Benefits and Risks of Insulin Pumps and Closed-Loop Delivery Systems
A case study in reverse causality and moral hazards
Listen to this really fun AI-generated audio summary of this article.
At a recent T1D conference I attended, a speaker spoke about a child whose A1c was over 10%, and time-in-range (TIR) under 20%. Her parents were gripped with anxiety. So, their endocrinologist put her on an automated insulin pump, and immediately, her TIR went up to 70% within a week. By the end of three months, her A1c had dropped to 7.3%.
The crowd gasped and applauded.
Medical literature is rife with similar success stories, and insulin pump companies publish data like this constantly. Go to any T1D conference or trade show, and that’s basically the future.
If you’re a T1D, I’m sure you’ve heard these stories, or are one yourself.
Quick recap of the system: A closed-loop insulin pump uses software algorithms that read glucose levels from a CGM and administer insulin depending on glucose levels. When your levels rise too high, more insulin is administered; when levels are too low, insulin infusion stops.
Indeed, AID (automated insulin delivery) systems are all the rage, and the greatest beneficiaries are those whose A1c levels prior to using them are north of 8-9%, which is fantastic. Because this mostly represents children, teens, and adults with either the inability or unwillingness to self-manage their T1D, AID systems have been praised as being the safety net for a large number of users. In fact, almost all scholarly articles that praise AID systems cite studies showing the enormous benefit to this cohort of users.
But, as I always caution readers, it’s not that simple. The vast majority of T1Ds are not so disengaged that they are able to see similar benefits, largely because most are at least partially engaged, so they aren’t that far out of control. For them—or, as we’ll see in larger stats later, most people—few people see A1c levels outside of the mid-7%, regardless of where they started. There are outliers in the high 6% range, but as we’ll also see, these are highly engaged users.
In short, the 7.5% range (+/- .5%) is the hard, upper limit on performance for automated systems. Moreover, physiological limitations make this an upper limit on how well they can ever do. (We’ll get into those physiological limitations later.)
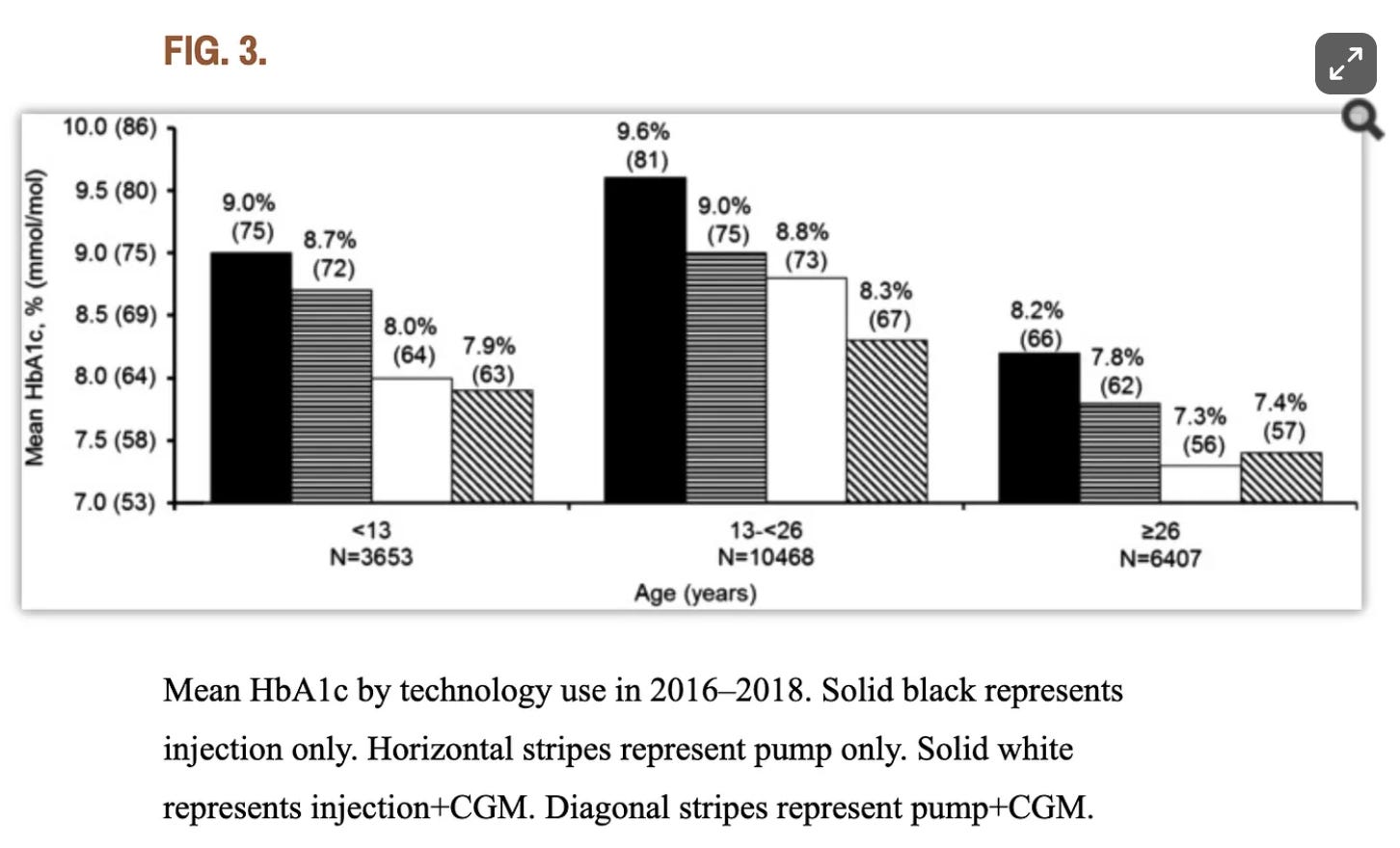
And medical literature validates this upper bound: Population-wide data that includes a much wider demographic than just the worst-controlled T1Ds continues to show that AID and MDI users achieve largely identical outcomes since at least 2018. That year, a research study by the T1D exchange found that, for adults over 26, “there isn’t a substantial difference between A1c outcomes for those who manually take insulin injections compared to those who use pumps,” including closed-loop insulin pumps. Below is a figure from that study. (The age group on the far right are those >26.)
This is not a critique of AID systems by any means! For those who are unable or unwilling to self-manage their T1D, these are great.
But for everyone else, let’s differente between association and causality. T1D outcomes are not due to the technology, it’s due to user engagement. The more engaged the user with the system—that is, announcing meals, exercise, and other details—the better the glycemic control. The pump isn’t performing better, the user is.
The moral hazard of these systems is the belief that the systems are doing the hard work. Indeed, these present ethical concerns, as discussed in the article, A critical review and analysis of ethical issues associated with the artificial pancreas, published in the journal PMC, 2018:
…the adoption of these systems involves complex questions about “patient agency“ and reliance on automation. If the technology makes the sedentary life “easy,” the path of metabolic adaptation becomes only “harder” by comparison.
This article aims to tease out the details behind these numbers and consider the longer and wider implications therein. We identify these key criteria:
There’s a difference between “target” glycemic ranges and “healthy” ranges. So, when people talk about what AID systems can achieve, that isn’t necessarily healthy.
Even then, not everyone is able to achieve these ranges. Who does and who doesn’t is essential to understand.
Insulin Pumps (regardless of automation) accelerates lipodystrophy, which aggravates the rate of insulin absorption. This unpredictability emerges gradually over time, yielding progressively worse glycemic control. The algorithms in automation cannot take this into account, and can exacerbate conditions.
Food absorption variability gets progressively erratic the longer one has T1D. Even if you announce meals, the system will assume it wants to release a progressive amount of insulin according to its algorithm, but it cannot possibly know how much food has actually been absorbed. For details, see my article, Food Absorption Variability and Treating Hypoglycemia.
Let’s take these individually.
Glycemic Targets
The first consideration—and arguably the most important one—is whether an AID system can help you achieve healthy glucose targets. The American Diabetes Association recommends an A1c level <7% and a time-in-range target of >70%, but those levels are not “healthy” ranges—rather, they are what medical care teams discerned to be achievable targets for most patients. Big difference.
For the past few decades, medical literature finds that socioeconomic factors are the greatest determining factor of success, not technology. Care teams use A1c of 7% and 70% time-in-range because most people believe they can hit those targets. Physiologically speaking, however, A1c levels of 7% are still quite unhealthy—long-term complications happen at those levels, too, according to the paper, The association of chronic complications with time in tight range and time in range in people with type 1 diabetes: a retrospective cross-sectional real-world study.
Having an A1c of 7% for 20-30 years is going to impose a great deal of irreversible harm on the cardiovascular system.
Automated delivery systems ideally get people into the mid-7% range, it presents a moral hazard: To get the most healthy outcomes, the user must to be engaged, and it’s hard to be engaged when “automation” is designed to disengage you.
For example, automated systems may calculate a reasonable dose for the food you ate, but only if you tell it the food you ate, or what you’re about to eat. Under those conditions, the pump will do better. But if you don’t tell the pump this information, it doesn’t make any adjustments till your glucose levels start to rise. And by then, the pump is playing a game of perpetual catch-up.
Even the pump companies themselves will say this: They can’t do as well by guessing—even with their advanced algorithms—than if you engage with the system. In Medtronic’s documentation for the MiniMed 780G system, they state:
“Carb counting is an essential component of diabetes management … and is still very important in automatic insulin delivery systems as per system design.”
The more you are engaged with your system, the better it will perform. But the system is not really automated anymore; it’s now in “semi-automated” mode. And that’s good! Those who perform the best are those who don’t even use the automation at all, only because you know what’s coming faster than the algorithm can figure it out. In other words, whatever it sees now was already visible to you far sooner. You just had to look.
Physiological Barriers
There are two more factors that keep automated systems from performing well: variability of insulin absorption AND food absorption. These are summarized in my article, The Insulin Absorption Roller Coaster and What You Can Do.
In short:
The longer one has T1D, the tissue where insulin is administered generates an inflammatory response, affecting the way insulin is absorbed. This is called lipodystrophy. Insulin pumps are far more susceptible to this than MDI (multiple daily injections), simply because infusion sets are delivering days worth of insulin in a single location, accelerating lipodystrophy. What used to be a rather smooth, predictable rate of absorption, insulin becomes increasingly less predictable in ways that are entirely chaotic. There’s no way for an automated system to know whether the insulin in infused an hour ago is still there, or has been fully or partially absorbed. All of its calculations rely on a set, predictable absorption rate.
Similarly, years of elevated glucose levels affect “gastric emptying”, the rate in which your stomach digests food. You may have eating a particular meal that you expect to absorb at a particular rate, but the longer you have T1D, the more volatile the “gastric emptying” process is. Again, you can tell the pump that you ate a pizza, and it can guess as to how to pace out the dosing to account for it, but if you have any variability—faster or slower—that algorithm will be wrong.
Put the two together, and you’ve got an entirely unpredictable situation. Any unexpected DIFFERENCE in the time when insulin is administered and when you EXPECT it to take effect—or when food is eaten and when you expect it to absorb—can result in unexpected highs, lows, confusion, rage boluses, panic eating, and more.
These dysfunctions explain one of the leading limitations for automated insulin pumps: No matter how much you analyze data and use historical context to predict insulin dosing, variability in food and insulin absorption is too stochastic to reliably factor into equations. The ability for algorithms to perform is capped by this randomness that only gets worse as glycemic control gets worse.
This is why those who perform the best with automation are children and recently diagnosed individuals, as they haven’t yet developed enough of these physiological dysfunctions to cause lipodystrophy or food absorption problems.
It’s also the case that this cohort of users also haven’t experienced T1D for long enough to develop good self-management techniques, so they’d already perform poorly on their own.
This is why almost all studies you see that show the benefits of insulin pumps are always using idealized candidates: People who are either unable or unwilling to self-manage themselves and who have not developed food or insulin variability.
Medical literature reviews
These hurdles keeping AID systems from improving is validated from peer-reviewed papers published in medical journals over the years.
One example is this article from the December, 2022 issue of Diabetes Care titled, “Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association.” The finely detailed analysis says that, so long as insulin and glucose levels have to use interstitial tissue for both getting glucose levels and administering insulin, the already-chaotic nature of glucose movements cannot be easily predicted.
A year earlier, this 2021 article published in The Lancet titled, “Opportunities and challenges in closed-loop systems in type 1 diabetes,” enumerates largely the same problems.
Go back even further, such as this analysis from 2015 titled, “Combining glucose monitoring and insulin delivery into a single device: current progress and ongoing challenges of the artificial pancreas.” As you can probably guess, the conclusions are largely the same: interstitial tissue and chaotic glucose patterns cannot be overcome with predictive algorithms.
Indeed, google scholar finds similar peer-reviewed reviews dating back to the year 2000 from researchers, scientists, T1D associations, and working groups, and they all come to similar (if not identical) conclusions: Unless we can get around the barrier of interstitial tissues for sensing glucose and delivering insulin, not a whole lot of improvement can ever be realized beyond current AID performance.
Now, again, this doesn’t mean AID systems are not useful, or even good. For many people who can’t achieve those levels on their own, they’re great. So, they should use them, right?
Well, some say that the other benefit of an AID system is that it can avoid hypoglycemia by turning off insulin delivery when glucose levels drop too low. That’d be good in theory, but reality shows otherwise. In a study published in the June 2024 issue of Diabetes Care is a paper titled, Severe Hypoglycemia and Impaired Awareness of Hypoglycemia Persist in People With Type 1 Diabetes Despite Use of Diabetes Technology: Results From a Cross-sectional Survey. The authors collected data from 2,074 T1Ds, half of whom used AID systems, whereas the rest were non-AID pump systems. Among all, “only 57.7% reported achieving glycemic targets of A1c <7%, but the number of severe hypoglycemic events from automated systems reached 16% for those using AID systems, versus 19% of non-AID pump users.”
And there lies the moral hazard: The attraction of an automated system keeps people from engagement, thereby leading to worse outcomes. In fact, a study from the T1D Exchange that examined data over eight consecutive years found that even though pump use increased from 57% in 2010–2012 to 63% in 2016–2018, the authors found that “the average A1c overall had risen from 7.8% to 8.4%,” largely because of disengagement.
So, there’s your choice: Convenience vs. healthy glucose levels. Pick One, because you can’t have both. (Parents: Do you have a transition plan for your kids to one day learn about T1D rather than rely on automation?)
Of course, if you are willing to watch your CGM and proactively administer insulin and/or carbs in order to preemptively prevent glycemic excursions, you’re awesome! True, not everyone can or will do that.
Technology Considerations
Another concern about insulin pumps is hardware malfunctions. I cover the topic more thoroughly in the article, “Conditions Where Insulin Pumps May Not Deliver Intended Doses,” which delves deeply into the subject. Among the many citations provided, the one that applies most in this context is a paper published by Jan S. Krouwer titled, “More Focus is Needed to Reduce Adverse Events for Diabetes Devices.” Krouwer reviewed publicly available reports from the Food and Drug Administration’s (FDA) search tool and found that “the percent of adverse events due to diabetes devices as a percentage of adverse events from all medical devices increased from 20.4% in 2018 to 30.5% in 2019 and is the largest contributor of any medical device.”
Since that article was written, the number of adverse events has skyrocketed given the advent of AID systems. This chart, from 2022, shows the number of adverse events that resulted in hospitalization from insulin pumps.
Even researchers are surprised by this. In the June 2024 issue of Diabetes Care is a paper titled, “Severe Hypoglycemia and Impaired Awareness of Hypoglycemia Persist in People With Type 1 Diabetes Despite Use of Diabetes Technology: Results From a Cross-sectional Survey.” The authors collected data from 2,074 T1Ds, half of whom used AID systems, whereas the rest were non-AID pump systems. Among all, “only 57.7% reported achieving glycemic targets of A1c <7%, but the number of severe hypoglycemic events from automated systems reaching 16% for those using AID systems versus 19% of non-AID pump users.”
Another valid consideration is whether T1Ds like and want to use a pump more than insulin pens. This is a phenomenon known as the Christmas Tree Effect, where users’ attraction to (and satisfaction of) new technology is often driven by the illusion that it is better than the older model/method, and it has lots of “bells and whistles.”
A Christmas tree is more fun if you just add more lights. And make them blink. Even better if they blink to Taylor Swift. Imaging an insulin pump doing that.
An example that illustrates this is the paper, Glycemic Outcomes in Adults With T1D Are Impacted More by Continuous Glucose Monitoring Than by Insulin Delivery Method: 3 Years of Follow-Up From the COMISAIR Study. The authors showed that insulin pumps – both automated and not – initially spurred engagement with users, but tapered off quickly, and ended up having little effect on overall glycemic control versus MDI.
But here’s the catch: The better outcomes were restricted to those who wore CGMs.
For that matter, the rise of CGM use has mirrored the rise of pump use (automated and not), which also leads to the impression that it’s the pump that drives improved outcomes, when in fact, CGM use is the strongest contributor of improved glycemic control over any other technology or method of insulin intake.
To be clear, those on MDI are subject to the same errors and challenges of engagement and self-discipline—a problem that’s difficult for ALL T1Ds—but at last they’re are not under the illusion that an insulin pen will do their work for them, or that it will relieve them of work. Pen users know that they have to be the ones in control of their T1D management. Over the course of time, MDI users learn better than pump users do.
An article published in the June, 2024, issue of Diabetes Care magazine titled, “Association Between Treatment Adherence and Continuous Glucose Monitoring Outcomes in People With Diabetes Using Smart Insulin Pens in a Real-World Setting,” the authors analyzed data from 3,945 T1Ds that use smart insulin pens (NovoPen 6 or NovoPen Echo Plus) and CGMs. They found that those who pay close attention to their daily management (as measured by reliably administering meal-time bolus injections) were able to achieve better A1c levels that are comparable to—or better than—AID systems. The key, of course, is that this group remembered to do meal-time boluses. Is that a big ask, to remember to bolus for meals? Hint: The more engaged you are, such as watching your CGM more frequently, the far less likely you are to forget to bolus for a meal.
User demographics and insulin pump outcomes
Perhaps the most important detail to consider about pump users is demographics. Given the high expense of pumps and CGMs (and the education and medical infrastructure to deliver them), pump users tend to be one or more of the following: They’re well-insured, come from higher socioeconomic households, have greater access to medical care, have higher income levels, and/or are typically more educated.
Perhaps most importantly, pump users tend to come from families that promote healthy lifestyles.
The more any of those conditions apply, the more likely that person is to be engaged and informed about managing T1D, the factor that outweighs all other aspects of T1D management.
The following paper, Racial, ethnic, socioeconomic disparities in insulin pump use have persisted over 20 years, breaks down this data accordingly:
“insulin pump use was 67% among non-Hispanic whites, 41% among Hispanics, 29% among Blacks, and 46% among other racial and ethnic groups. In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000 to 74,999, 51% with $25,000 to $49,999, and 41% with less than $25,000, used the pumps.
Many attribute this as being the inequality of our healthcare system. That is, people who are white, rich, and educated have better access to technologies like automated insulin pumps.
True, but again, it’s not the pump doing the real work; it’s all the other factors.
To test whether this is the case is to review trials where subjects’ A1c levels are measured before and after introducing them to pumps and then evaluating their outcomes according to the various socioeconomic groups.
The paper, Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis, analyzed dozens of random controlled trials (RTCs) among a wide range of socioeconomic groups, and also factored studies involving T1Ds who exercise. The authors concluded that pump users were only able to achieve a 1.07% improvement over control groups who didn’t use an an insulin pumps (and did use CGMs), irrespective of their background.
One can get even more granular still: In a 2021 study titled, Predictors of the effectiveness of insulin pumps in patients with type 1 diabetes mellitus, the authors conclude that the subjects whose A1c levels were >8% going into the study saw an improvement of 0.9 ± 1.2% when started on insulin pumps, whereas those whose A1c levels were <8% saw no benefit to pumps at all, irrespective of age, race, and income levels.
This is another example of statistical significance, but clinically irrelevant.
Lifestyle and other factors
Lastly, there are those who claim that, even if pumps offer no clinical benefit, the lifestyle benefits make them worthwhile.
Don’t take this the wrong way, but are they really having a better lifestyle with pumps? Fortunately, there are RCTs that study that too.
An interesting paper is The DIAMOND Randomized Trial, which initially aimed to assess the relative difference in costs associated with pumps versus MDI therapy. The authors found that pump users’ lifetime costs are $112,045 greater than those on MDI.
But researchers unexpectedly found that pump users’ quality-of-life factors decreased by 0.71 compared to MDI, and (even more unexpectedly) life expectancy dropped by 0.48 years (based on higher risk of DKA, among other risk factors that rose). Minimal differences from MDI to be sure, but these numbers certainly didn’t improve, as pump advocates would like to believe.
The reduction of efficacy AND the higher costs of insulin pumps (compared to pens) are two primary reasons why insurance companies are reluctant to cover insulin pumps.
Similar conclusions are found in multiple studies compiled in the paper, Systematic literature review: quality of life associated with insulin pump use in type 1 diabetes. By assessing a much larger set of trials, the authors concluded that there was little change in quality-of-life outcomes for most users due to a subtle psychological bias: Pump users really wanted them to work better, and in so doing, they believed they were. This makes “actual” satisfaction hard to assess, which brings us to the realm of psychology, especially around technology.
In the BMJ paper, Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review, the authors aimed to summarize incidence and prevalence of T1Ds who were admitted to hospitals for DKA, and sort them into different subgroups (age, sex, geographical region, ethnicity and type of insulin administration). The paper is excessively long, as the reviewers started with 1082 separate articles in order to characterize each of the criteria. Among the many citations, a clinic in Colorado stood out, where all the patients admitted with DKA used pumps—none were on MDI. Other citations are listed below:
In the paper, Insulin pump therapy is associated with higher rates of mild diabetic ketoacidosis compared to injection therapy: A 2-year Swedish national survey of children and adolescents with type 1 diabetes, the authors found that 85% of admissions were pump users vs. MDI.
In the paper, Severe Hypoglycemia and Diabetic Ketoacidosis in Adults With Type 1 Diabetes: Results From the T1D Exchange Clinic Registry, researchers performed a cross-sectional analysis from the T1D Exchange clinic registry at 70 U.S. endocrinology centers, and found that 2972 incidents of DKA were associated with pump use, versus 2034 for those on MDI (roughly 47% greater risk for pump users).
In the paper, Causes of diabetic ketoacidosis among adults with type 1 diabetes mellitus: insulin pump users and non-users, researchers conducted a prospective observational study between January and June 2019 at the Cleveland Clinic Fairview Hospital, and found that, among patients admitted with DKA, 55% of the cases were pump users.
The list of similar findings is extensive.
Many people assume that DKA from insulin pumps is largely due to device malfunction, but this isn’t always the case. A more prevalent reason for DKA is user error. In the paper, Risk and Relevance of Insulin Pump Therapy in the Aetiology of Ketoacidosis in People with Type 1 Diabetes, the authors found that “an overwhelming majority were those on insulin pump treatment (93%)”, and added, “Overall, patient errors caused more DKA cases than device malfunctions.”
Imagine that: user error on a device that should be either automated or semi-automated, and where people are enamored with the technology, and who prefer to use them over much simpler insulin pens. The authors of this paper, DKA Prevention and Insulin Pumps: Lessons Learned From a Large Pediatric Pump Practice, were more blunt: “Most events could have been avoided if users followed standard troubleshooting guidelines.”
According to this T1D Exchange report, the authors state that, although the use of pumps increased from 57% in 2010–2012 to 63% in 2016–2018, “the average A1C overall has risen from 7.8% to 8.4%.” The authors conclude that A1c levels are directly correlated to user engagement. For those who relied on the pump to manage their disease for them, they fell further behind than those who bothered to learn on their own.
This sentiment is similarly expressed by the authors in a 2022 review of closed-loop systems in the Diabetes Journal article, Diabetes Technology: Standards of Medical Care in Diabetes—2022, where the authors wrote:
The most important component in all of these systems is the patient. [...] Simply having a device or application does not change outcomes unless the human being engages with it to create positive health benefits. [...] Expectations must be tempered by reality—we do not yet have technology that completely eliminates the self-care tasks necessary for treating diabetes.
When it comes to engagement, this is one of the primary advantages of MDI. The physical act of injecting insulin makes one keenly aware of what the patient is doing. This is an example of The Hawthorne Effect, a phenomenon where people modify their behavior (usually positively) when they know they’re being observed. Observing yourself is the base case of the Hawthorne Effect.
In a study titled, The Importance of the Hawthorne Effect on Psychological Outcomes Unveiled in a Randomized Controlled Trial of Diabetes Technology, study subjects were exposed to a series of tasks associated with T1D management. The authors found that T1Ds who exhibited fear of hypoglycemia (FOH) saw a significant reduction in FOH, simply because they were being closely watched, even though there was no intervention whatsoever in how they managed their disease. They simply did what they knew they should do on their own. (All the other studies cited in this article do not have subjects “closely watched.”)
Similar outcomes have been shown for T1Ds who are told to log carbohydrates, exercise, and yes, manually take insulin. When you have to stop and think about calculating insulin and carbs, the act of what you’re about to do becomes more present. You proactively think to yourself, “Do I log 40g or 60g for this glazed donut that I know I shouldn’t be eating?” Believe it or not, the physical act of logging those carbs and taking that insulin causes many to be more engaged and deliberative in their T1D choices. Many people–including me–often choose not to eat that glazed donut. Sigh.
Summary and Commentary
I have lived with T1D for over fifty years, so I can attest to the fact that the disease is grippingly difficult to live with, let alone self-manage. Any technology that can ease the burden—even in the slightest—is a blessing. And this is why I struggle with the concept of automated insulin pumps: They are essential for those who are either unable or unwilling to manage their own care beyond a certain threshold, especially the young or those with cognitive impairment. But for those who are able to take care of themselves, one must remain as engaged as possible with the nuances of daily T1D management. One of the risks of automation is disengagement: failing to learn self-management.
The state of closed-loop technology today is, sadly, as good as it can possibly get, entirely because the randomness of insulin and food absorption variability, which gets progressively worse over time, cannot be factored into dosing algorithms.
It will be more interesting to see what the medical outcomes look like over the next five and ten year cycles. It won’t be the first time that a technology that was once thought to be life-changing turns out to be less than glorious.




Once again, you've written an extremely thought-provoking article. Thank you for putting this together!
I've had T1D for 45 years. I use Control IQ and I (1) love it and (2) don't disagree with your conclusions in the least. I'm meticulous about bolusing for food and high blood sugars. Without that effort, the pump wouldn't be effective at all.
Why do I love it? For me, it's COMPLETELY eliminated overnight lows since the day I switched to CIQ. I used to run high at night out of fear of those overnight lows. No longer! I wake up every morning at 110 mg/dl. This is the one area where closed loop tech is (understandably) excellent. When a diabetic is asleep, the pump can simply release basal insulin, adding more or giving less as needed. Without meals or exercise or stress to worry about when the patient is asleep, it's pretty hard for CIQ to mess up. That alone is worth it for me. But nothing I'm saying contradicts your conclusions.
Thanks again. I look forward to reading each of your posts.
Proofreading Police! 😉
“Technically, such a system a highly“
You’re missing a verb, perhaps “is”?
“Another way to test that is by measuring A1c levels before and after introducing people to pumps and measuring their outcomes afterwards (while including subjects from diverse social, racial and economic subpopulations). This metaanalysis (literature review) of dozens of RTCs had these aims in mind, and also included”
Please, sir, what are RTCs?
Policing over. Again, thank you for an interesting article. I always learn something from your posts. I'm a Metronic user and I got a new pump over a year ago. They gave me a box of sensors and a chance to try out this new 780G system. Well, I have a problem with rising blood sugar after I get out of bed. I like to eat right away, so first thing I do is check my numbers and enter carbs for breakfast. However, auto mode thought that my rising blood sugars were from eating, but I knew they weren't. So I had to exit auto mode, bolus in manual mode, and then go back to auto mode a few hours later for the rest of the day. I learned from a forum that I could "phantom bolus" to correct, but it just seemed a hassle. It was way easier to set my Basals where they needed to be. Plus, I would have to pay out of pocket for the sensors, when the Libre 2 was covered by the government.
I missed my old pump, because the new one was specifically designed to be used in auto mode and manual mode requires seemingly endless button pressing. Unfortunately, I forgot to take that old pump off when I went swimming in the sea. "Critical Error!" ;-) So, back to my "back up", newer pump.
My 2 months on the Medtronic "closed loop" system was very frustrating and my time in range numbers were worse. I can see where it would be advantageous for someone who was hypo unaware, especially at night, but it just wasn't working for me.
I look forward to your next article.