Conditions Where Insulin Pumps May Not Deliver Intended Doses
Insulin pump tech is complicated, and human physiology doesn’t make it easier.
Listen to this really fun AI-generated audio summary of this article.
One of the most maddening aspects of managing T1D is the erratic and unpredictable variability in the absorption and responsiveness of insulin, which can wreak havoc on glucose control. But a related factor that isn’t really considered very often—if people even know about it—is the possibility that insulin pumps may not always deliver the amount of insulin they’re supposed to.
This recently came up in an email from one of my subscribers:
“I'm the parent of a newishly diagnosed (6 months ago) T1D 6 year old. We started on MDI, and now we're on Omnipod 5 in manual mode, basically used as fractional injections. But we've noticed that each change-of-pump (every 3 days) seems to result in different insulin sensitivity (and therefore carb ratio) unlike MDI, making it hard to be predictive.
It seems that the pump site, pump day-age, and recency of site usage all affect the carb ratio. At its best, the pump results in equal sensitivity as MDI, but can get as low as 1/2, e.g. if a specific site was overused. The ability to rotate MDI sites seems to eliminate this.”
Given the child’s age, time of T1D duration, and other factors (diet, exercise, etc.), we were able to eliminate possible culprits, leaving the possibility that maybe the pump wasn’t actually delivering the amount of insulin it was supposed to.
Investigating medical literature, we discovered published papers on similar situations dating back to 1994, citing mechanical problems with insulin pumps that have long been known, but rarely discussed—or addressed. In fact, the technical inserts included in the product packaging for all pumps provide warnings on insulin delivery variability, though not to the degree of detail provided here, nor with explanations on why.
This article aims to explain the mechanical deficiencies with pumps described in published medical journals, what can be done to fix them, and whether that’s even enough. (Again, nothing in T1D is that simple, so don’t expect miracles.)
But before you gaze suspiciously at your own pump, everything here should be taken into a much broader context: You may well be very happy using an insulin pump, and don’t see any reason to be concerned. What’s more, if your own A1c levels—or those of your child—are consistently north of 8%, then automated insulin delivery (AID) systems may well be a wise choice, despite some of these problems.
All that notwithstanding, the details described in this article should be considered in any context.
Injection pressure: A shortcoming with insulin pumps
What was most likely happening to the child in the email correspondence is that insulin itself was causing interstitial tissue to react in ways that presented a physical barrier to the pump’s ability to provide enough pressure to deliver the intended amount of insulin. This phenomenon is detailed in the 2022 peer-reviewed paper, “Insulin induces a progressive increase in the resistance of subcutaneous tissue to fluid flow: Implications for insulin pump therapy.” Here, the authors set up a study consisting of thirty T1Ds wearing two Accu‐Chek Spirit Combo insulin pumps. One pump used actual insulin, while the other used an insulin diluting medium. The pressure in all the pumps was measured as the days progressed, and the authors found that the pressure associated with pumps using insulin had dropped significantly, versus pumps using the diluting medium, which remained constant.
The pump itself is not that important insofar as this study is concerned, since the aim of the study was to investigate insulin’s impact on subcutaneous tissue and its effect on pump mechanisms. The authors found that insulin causes a proinflammatory response in subcutaneous tissue that inhibits fluidic flow, which the pump is neither sensing or compensating by increasing the pressure needed to infuse the intended amount of insulin. As the days go by, progressively more insulin causes more inflammation, resulting in less insulin delivered because the pump did not apply enough pressure to push the insulin through the affected tissue.
Note that this is not an example of insulin resistance, which is a condition where insulin is unable to reach cells after it’s already in the bloodstream, which can happen due to a large number of factors unrelated to this present situation.
Nor is this an example of lipodystrophy, a phenomenon characterized by the local accumulation of dense fat tissue at insulin injection sites in a manner where insulin is not absorbed into the bloodstream in a constant, predictable manner. While that can also present similar problems, lipodystrophy is the result of years of insulin exposure, not days. The child in this case only had T1D for six months, so lipodystrophy wouldn’t have had time to develop (though this is important for long-term T1Ds to know about).
The mechanisms of action associated with this particular pro-inflammatory response is detailed in the paper, “Therapeutic Insulin Analogue Concentrations at Infusion Sites Enhanced the Pro-Inflammatory Response and Apoptosis in an In Vitro Macrophage-Material Interaction Model.” Here, the authors identify a number of proteins that are activated in response to insulin that causes the inflammation, and remain active so long as insulin continues to be infused. Again, these proteins are unrelated to either lipodystrophy or insulin resistance.
The pump used by the child in the email exchange was an Omnipod 5, which is a tubeless pump. But pumps that use tubing suffer an even more erratic problem with where the tubing is relative to the infusion site. In the article, “Siphon effects on continuous subcutaneous insulin infusion pump delivery performance,” the study authors monitored the rate of insulin infusion through the tubing and into the infusion site, and found that when the infusion site is below the tubing, only 86.7% of the intended amount of insulin was infused, versus 117.0% of insulin was delivered when the infusion site was above the tubing. The variability is due to fluidic dynamics of the insulin traveling upwards or downwards.
Since few people are aware of either of these phenomena, they are rarely considered if they happen to experience odd glycemic behaviors. Rather, they’re quick to blame other things that may not necessarily be a contributing factor. For example, I predicted that the child’s endocrinologist would blame cortisol for the erratic nature of the insulin-to-carb ratios, and in fact, the parent confirmed that’s exactly what the endo said. (Cortisol is almost always blamed whenever anything unexpected and unexplained happens with T1Ds.)
The problem in this case is that the pump is not very accurate in how accurately it pumps insulin—a hardware problem with the syringe-like mechanism that pressurizes the fluids to move into the body.
This situation raises another, more serious questions: What other mechanical problems do insulin pumps have? And how serious are the health risks they pose? To answer those, we first want to step back and use a wider lens to define these areas of interest.
“Adverse Events”
In the medical world, an adverse event is defined as any unintended negative outcome that occurs as a result of medical care, such as unwanted effects from a medication, mistakes made during medical procedures, equipment malfunction, or any other situation that can be attributed to the intervention itself.
To understand the degree in which insulin pumps cause adverse events of any kind—not just this particular one—Jan S. Krouwer, an independent researcher, published a peer-reviewed paper titled, “More Focus is Needed to Reduce Adverse Events for Diabetes Devices.” Krouwer reviewed publicly available reports from the Food and Drug Administration’s (FDA) search tool and found that “the percent of adverse events due to diabetes devices as a percentage of adverse events from all medical devices increased from 20.4% in 2018 to 30.5% in 2019 and is the largest contributor of any medical device.”
Refining the data further, a paper by Robert D. Butterfield and Nathaniel M. Sims used the same FDA database as Krouwer, but limited their search to types of insulin pumps between 2013 and 2023.
In this chart, each color is associated with a three-letter code that the FDA uses to correspond to either a specific manufacturer’s device, or a class of device. (Green refers to the aggregate number of events associated with only the data collected, not the entire database from the FDA’s portal.)
Note each pump manufacturer can designate their own code (or set of codes) when they submit an application for approval, and are not obligated to use existing codes of other products or categories where there may be crossover. For example, OZO (gray) refers to the t:slim X2 Insulin Pump with Basal-IQ Technology, a single product that does not use the other product codes, despite the fact that it could have also used the generic LZG (blue), which refers to any general insulin pump. (Chances are, few newer products are using the LZG code, leaving it as a legacy code for older devices, which fewer people are using.)
OZP (orange) refers to an automated insulin pump, which captures more of the more recent offerings, especially as manufacturers are moving in that direction.
QFG (lighter blue) refers to a pump with an “alternate controller,” such as a phone, which could encapsulate a large set of devices.
OYC (red) refers to an insulin pump that is “used with an invasive glucose sensor,” such as a CGM, which is increasingly more common, but again, fewer manufacturers are choosing to use that code.
One can imagine that all three of the codes OZP, QFG and OYC probably comprise most of the more modern AID systems, but again, the researchers did not delve deeply into the database to confirm that.
Importantly, Krouwer pointed out in her paper that the number of adverse events in the database may be vastly underestimated because many people are unaware that their experiences could be due to device malfunctions, so they go unreported. And even if they report them to their device makers, Krouwer says “it’s not clear that those are logged on the FDA’s portal.”
And yet, even with these underestimates, the total number of such reports exceeds all other medical devices, not just diabetes-related.
As for the type of adverse events associated with pumps, the authors filtered for insulin overdose leading to hypoglycemia, and insulin underdose leading to DKA. These are not just mild inconveniences.
Now, to be clear, the mechanical issues that cause these more serious events are unlikely to be solely due to the inflammatory response cited earlier. According to the paper by Butterfield and Sims, the two factors causing these more serious events are (1) “delayed detection and notification of occlusion without the user's awareness,” and (2) “the presence of air replacing insulin in the reservoir or delivery path” — both of which can lead to hospitalization.
Automated Insulin Delivery Systems
Before we look into those, we need to take a small tangent to recognize that automated pumps are generating an increasingly larger share of adverse events, and automation is driven by software, not hardware. It may very well be the case that poor algorithms may be responsible for some number of these events. But again, without proper hardware and software tracking—or regulatory standards by which to define and enforce such tracking—it’s hard to know.
An illustration of this comes from a study published in the June 2024 issue of Diabetes Care titled, “Severe Hypoglycemia and Impaired Awareness of Hypoglycemia Persist in People With Type 1 Diabetes Despite Use of Diabetes Technology: Results From a Cross-sectional Survey,” which showed that the number of severe hypoglycemic events from AID systems was 16%, compared to 19% of non-AID pump users.
While the 5% improvement between these groups is statistically interesting, it’s not that “clinically” relevant, largely for the reason cited above: without knowing the cause of these events, it’s hard to truly establish where things are going wrong.
Interestingly, the lead author on that paper is Jeremy Pettus, the host of the popular podcast, TCOYD (Taking Care of Your Diabetes), and is also a practicing endo, a T1D himself, and a leading advocate for AID systems. In an interview with the Diabetes Journal reporters for their own podcast when asked about the high rate of adverse events with AID systems in his own study, he said he was “shocked” that these systems performed as badly as they did, suggesting that considerably more work is required to improve AID algorithms. That may well be, but it may also be that the nature and diversity of errors (and randomness) within the physiological ecosystem of delivering insulin may be that no more improvement can be realized from where we are now. (I get into considerably more detail on this in my article, “Challenges Facing Automated Insulin Delivery Systems.”)
Ok—back from the tangent. Let’s get back to the mechanics of the pumps.
I know what you’re thinking: How are potentially faulty insulin pumps getting FDA approval?
Well… it’s not that simple. (As if you didn’t see that coming.)
FDA approval of insulin pumps
For manufacturers to receive FDA clearance to market and sell an insulin pump (or most any other kind of device), they need to (among other things) demonstrate in a clinical trial that the devices are “safe and effective.” This euphemistic-sounding term is more vague than what the general public thinks. Essentially, it boils down to conducting a clinical trial that involves two groups: a study group who wears the new device, and a control group who uses a previously approved device of a similar nature. E.g., an insulin pump. So long as the study group does not do worse than the control group, the device is usually granted approval and they are allowed to use the “safe and effective” terminology. (There’s more to it than that, of course. My article on how to better understand clinical trials for T1Ds is here.)
Wait, didn’t we just see there are lots of adverse events? Why can’t the FDA at least mandate minimum standards for mechanical operation to at least try to minimize adverse events?
To do that, they first need to establish standards, which are currently too generalized to be meaningful (or address these problems). This, despite the fact that in 2015 the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group made a series of recommendations that have not yet been implemented (even informally).
But what’s getting all the attention now is automation—that is, artificial intelligence—and it’s changing more rapidly than what legislation can keep up with, including the regulatory arm of the FDA. Trying to establish “performance standards” for insulin infusion automation is not likely to happen anytime soon. If you don’t believe me, just ask ChatGPT, though you’ll probably get a lot of misinformation in its response. Oh, sorry, the politically correct term is “hallucinations.”
User Error?
One element that device manufacturers often point to is that users themselves are not managing their devices properly because they don’t pay as much attention as they should. And that’s putting it graciously.
In the paper, “Risk and Relevance of Insulin Pump Therapy in the Aetiology of Ketoacidosis in People with Type 1 Diabetes,” the authors found that “an overwhelming majority [of DKA events] were those on insulin pump treatment (93%)”, and added, “Overall, patient errors caused more DKA cases than device malfunctions.”
That may seem like the device’s malfunction is not a problem—that the user just chose the wrong dosage, for example—but again, most people are unaware that devices can malfunction in these subtle ways, so it wouldn’t occur to them if there ever was a malfunction.
This notion is further considered in a separate paper, “DKA Prevention and Insulin Pumps: Lessons Learned From a Large Pediatric Pump Practice,” the authors were more blunt: “Most events could have been avoided if users followed standard troubleshooting guidelines” for devices that exhibited mechanical faults.
This raises a critical question: To what degree can or should we rely on USERS to recognize or even deal with mechanical problems? Remember, those users include children and [distracted] teens. Should we expect them to be aware of or even fix “mechanical malfunctions?” It’s hard enough just to get them to observe their CGM numbers between TikTok videos.
And you adults aren’t off the hook. I’ll bet have the readers here don’t even know how to change the toner in their inkjet printers! (Ok, I admit, it’s a toughie. Heck, even knowing what cartridges to buy is an exhausting experience.)
And yet, we expect people to troubleshoot an insulin pump?
Ok — your head is spinning. I can see your sugar must be low. Take a moment to recover.
Ready? Good, let’s get back on track: We know what the mechanical problems are, so can they be “fixed?”
Yeah, but set your expectations modestly.
The plot twist: a new device
As it happens, the authors of the paper cited earlier (Butterfield and Sims) have a background in mechanical engineering, particularly devices that work with gasses and fluids, so they built a subsystem to monitor long and short-term insulin flow accuracy, occlusion-detection time and pressure, and air management in the insulin reservoir of insulin pumps. Here are the parameters they aimed to track:
Insufficient pressure to infuse the intended amount of insulin into tissue (ie., the child’s experience)
Sensing blockage and not overcoming it (or setting an alarm), such as a kink in tubing.
Inadvertently delivering air that may reside inside insulin reservoirs.
Failing to detect and overcome site leakage.
They used this subsystem to test for mechanical weaknesses in three commercially available pumps, described below. And while they were at it, they also built their own proposed insulin pump, which they called a “new device” (ND) that has features that overcome the shortcomings listed earlier. They used the data from the tests of the other devices and their new device in their application to the FDA who then granted approval for their new device. (But don’t rush to buy it yet. As of this writing, Sept 2024, it’s not available.)
Again, their paper with all this data is “Performance of a Continuous Subcutaneous Insulin Infusion (CSII) Pump With Acoustic Volume and Flow Sensing in Simulated High-Consequence Situations.”
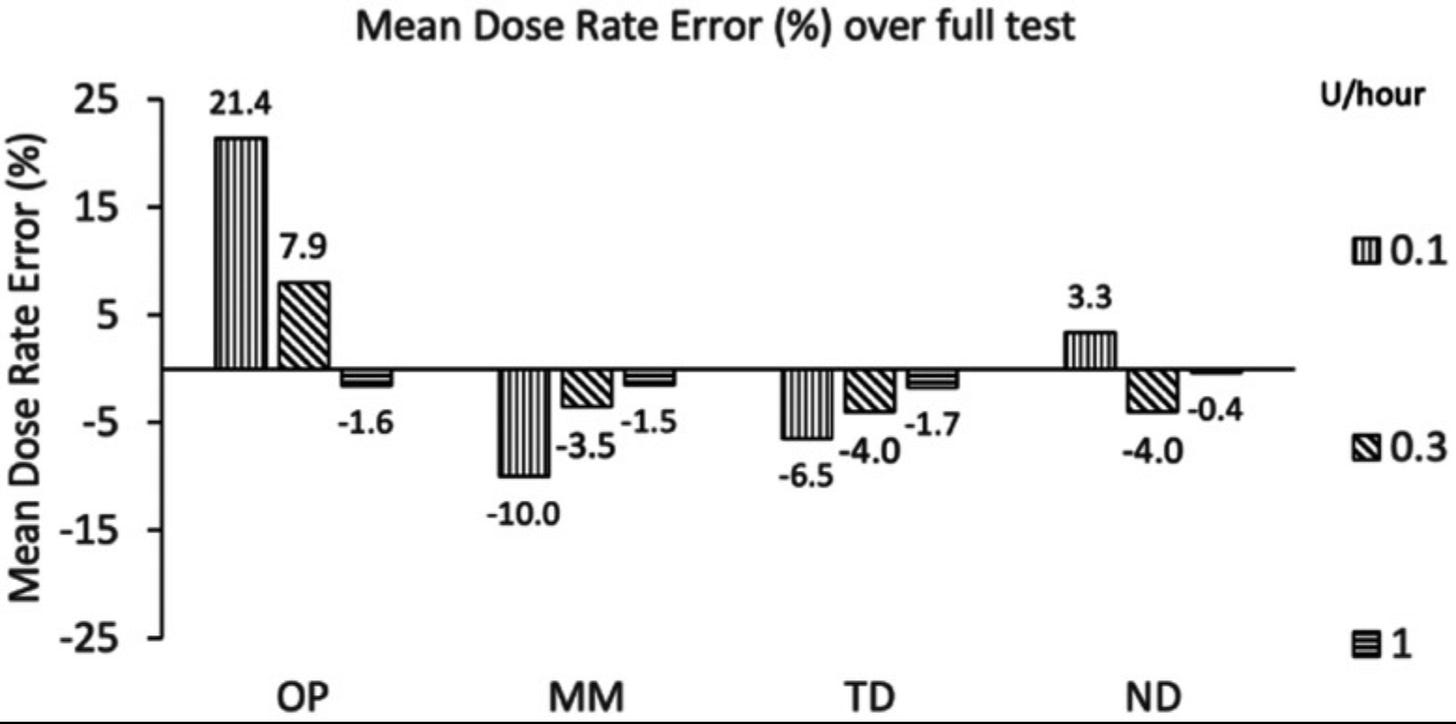
The graph below shows the mean dose rate error of these commercial pumps against their own.
OP=Insulet (Omnipod), MM=Medtronic (600/700 series), TD=Tandem Diabetes Care (t:slim X2), ND=new device
The first test explores basal insulin flow rate among each of the pumps, where the error rate reflects the difference between the intended dose versus the amount of insulin that actually came out. This is the part that controls the pressure needed to infuse insulin.
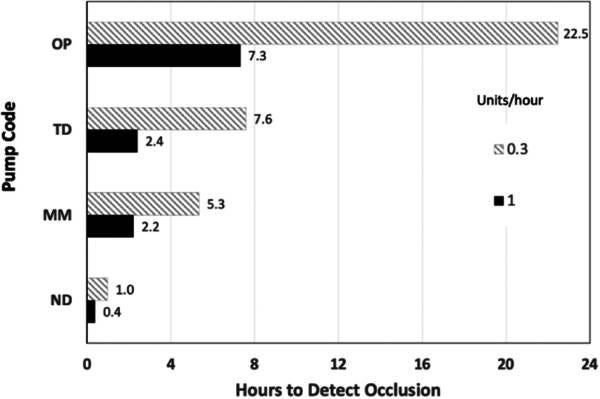
Another problem that occurs with pumps is the amount of time it takes for the pump to detect an occlusion that prevents insulin from being delivered, which can result in pressure building up to produce site leakage, a far more likely cause for DKA. Below is a chart showing how long it takes for the various pumps to detect occlusions.
Lastly, there’s the matter of air management, which can happen when users fill reservoirs with insulin. Air accounts for an even greater likelihood of adverse events, and worse ones at that. According to the paper:
“Each of the pumps was loaded with a full insulin reservoir that also contained 0.2 mL of air, primed and allowed to run at 15 U/h to empty. The data shows OP, MM, and TD pumps were able to deliver the reservoir air, without interruption or alarm, until the reservoir was completely empty, whereas ND continued to deliver fluid uninterrupted, neither alarming nor delivering the air placed in the reservoir.”
Speaking of air management, the industry has known about insulin pump delivery errors during airplane flights for decades, according to the paper, “Changes in Altitude Cause Unintended Insulin Delivery From Insulin Pumps,” published in 2011. Here, the authors measured the degree in which atmospheric pressure reduction causes unintended insulin delivery in pumps by bubble formation and expansion of existing bubbles. At the time, the authors found more than 50 cases of severe hypoglycemia from adults and children due to this phenomenon. They further point out that this phenomenon was first published in 1994 by Aanderud and Hansen, who demonstrated that insulin pumps delivered “more insulin than the set rate during decompression.”
The study that demonstrated the performance of both the commercial pumps and the “new device” prompts one to wonder: What’s this “New Device?”
It’s called The Twiist, and it was produced by a company called Sequel Med Tech, where one of the cofounders is Dean Kamen, who invented the first insulin pump in 1976. He also leads a team at DEKA Biosciences, which has expertise in manufacturing products like this. (Again, the Twiist is not available at the time of this writing, Sept 2024.)
According to Sequel’s website, the Twiist will be powered by AI from Tidepool to be used as an automated insulin delivery (AID) system.
While this may sound exciting, there are two things to keep in mind. First, the software that drives predictive algorithms in AID systems is still new and under rapid development. It’s not clear whether the software component is (or will) work as expected. While it’s good to fix hardware, the algorithms are what we need to really watch. (Did you read that other article I mentioned earlier? No? OK, here it is again.)
Secondly, even if pumps could overcome all the problems listed here—mechanical and software-related—the elephant in the middle of the room is insulin itself: It has properties that make absorption and efficacy subject to high variability, regardless of how you administer it. (Even non-diabetics have volatile glucose patterns, with high degrees of variability from one day to the next, even under tightly controlled conditions. For more on that, see my article, “Why Controlling Glucose is so Tricky.”)
Epilogue
This is not all bad news. There are many ways to maintain good glycemic control, despite these problems (and others unstated). As I mentioned at the beginning of this article, many T1Ds do very well with AID systems and pumps, notwithstanding these problems. Unfortunately, the reason they do is because, for many—children, teens—either cannot or will not manage their disease on their own. If your A1c is north of 9%, AID systems are great. If you’re between 7-8%, AID systems may not necessarily provide improvement—your mileage may vary. But if you’re able to manage your T1D on your own and achieve <7%, then you should probably stay the course.
I detail all these factors in, “Benefits and Risks of Insulin Pumps and Closed-Loop Delivery Systems.”
No matter what you do, keep in mind the golden rule of T1D: “keep an eye on your CGM numbers.” That’s the best way to know if something might be amiss and needs your attention.
Don’t become an “adverse event.”