Basal Insulin Reduction: A How-To Guide using “Active Learning”
Reducing and refining basal insulin profiles using controlled experiments improves glycemic control, reduces hypoglycemia, and yields healthier outcomes.
This article is the last of my four-part series on overbasalization, which the American Diabetes Association has identified as a major health concern for T1Ds, according to section 9.27 of their latest (2025) guidelines. The three specific signs of overly aggressive basal dosing are frequent and severe hypoglycemia, more difficulty in daily T1D management, and weight gain.
The aim of this article is to understand ways to reduce basal dosing to be in line with physiological needs.
The first article in this series explains the 50/50 rule—the assumption that 50% of your total daily dose (TDD) should come from basal dosing—and how recent research finds that it’s more complex than that. The previous article in this series, Basal Dosing: How Much Do We Need?, reviews that research, and it can be summed up in these three points:
The liver’s “autoregulatory” glucose production is not dripping glucose like a leaky faucet, as was once thought. The liver only produces glucose when food is not present (outside of things like exercise and other anomalous conditions). So, the glucose the liver produces—what basal insulin is supposed to cover—is not evenly distributed throughout the day when we eat. It’s weighted mostly at night. This means that most basal dosing is largely covering meals alongside bolus doses. This mixture is impossible to measure, making dosing calculations imprecise.
Even when the liver produces basal glucose, that rate of production not flat. It’s volatile, and occurs at different rates at different times, even in a fasting state. Accordingly, the insulin needed to cover it would not require a steady, flat drip. Therefore, a flat basal insulin formula or algorithmic “rate” for pump users would be out of alignment with actual, physiological needs. In other words, a basal ratio says nothing about the RATE of delivery.
Lastly, studies show that there are often insulin-free gaps in normal physiology. Accordingly, if there is insulin onboard during these physiological windows, it may likely trigger hypoglycemia, and that presents a whole new set of problems.
Suffice to say, our traditional understanding of T1D management does not align with physiological reality, nor is it reflected in current ADA guidelines (or what you hear from your doctor). This, despite the fact that all the studies that led to these discoveries were mostly published in ADA journals.
All this leads to the fact that ideal glycemic control is best achieved by “dosing as needed”. As we’ll see later in this article, this is exactly where automated insulin pumps are going today, and with great success under some (not all) conditions. For more, see my article, Medical Literature Analysis: The Performance Paradox of AID Systems.
But it also comes with one major caveat: A fully-automated system can only react to glucose levels after the fact. To perform better, they need to see the future. Specifically, what you’re about to do in the next 30, 60, and 90 minutes. Whether it’s food, exercise, sleep, or other events, it’s only when the user engages with the algorithm can it truly perform to its potential.
This is the case with MDI as well. When the user is similarly engaged—in fact, exactly as engaged—one can achieve the same or better outcomes as automated systems.
Learning how to be engaged is the aim of this article. It’s a process that I call the Active Learning T1D Boot Camp. This is where you use hands-on experimentation to discover your own personalized metabolic profile, which forms your ideal basal dosing strategy.
Note that this is not a way to pure people away from automation or their traditional self-management techniques. In fact, quite the opposite. It’s an immersive process that lets you truly understand yourself so that, when you return from it, you can improve upon whatever protocol you subscribe to, whether it’s automation or 100% MDI.
Many are initially intimidated by this because it demands considerable time and attention for about 2-3 weeks. Moreover, no one is going to sit beside you and answer questions, give guidance or opinions, or provide medical oversight. That’s simply not possible. If you need that—and there’s no shame in it—then you should seek the advisement of registered clinicians and diabetes educators. Learning the basics that they teach you is like training wheels for those who haven’t mastered learning to ride a bike on their own. It’s vitally important to be safe first. But sooner or later, like a bicycle, T1D is a solo act, and you need to spend your life making entirely your own decisions, in the moment, every moment.
In this context, the boot camp I’m about to describe should only be undertaken by those who are mature and experienced enough with T1D to make adjustments to their insulin regimen on their own. And that is not universally the case.
The payoff comes afterwards. The time you invest gives you an intuitive sense of your own, unique T1D profile that stays with you for life. You will naturally relax into your own state of automation. It becomes so second nature, you hardly know you’re doing it. While others struggle with self-management their whole lives, your small investment can bring you years of far lower anxiety and better glycemic outcomes.
Either way, there’s something to learn by simply reading this article, as it’s part of the self-education journey.
Total Daily Dose, Basal Ratios and the Big Tradeoff
Let’s start with a reference point for ideal conditions. In an interview, Dr. Ralph DeFronzo, a leading expert in diabetes research, noted that a healthy, lean T1D typically requires between 35-40 units of insulin per day to maintain normal glucose control. This is called the Total Daily Dose—TDD.
Put this into context for the general population: A T1D Exchange study of 1,969 adults (mean age 45 ± 13 years) showed mean TDD between 44-49 units for those with “stable” weight (not technically obese), and 49-61 units for those whose weight was ≥20 pounds over baseline (obesity).
This discrepancy is revealing on its own. One can infer that, with the fact that 37% of T1Ds are now considered obese (according to a 2023 Lancet article), many T1Ds—particularly those with elevated TDD and excess weight—should be reducing their basal dosing.
Ok, reduce basal ratios to what?
Here’s where the situation gets interesting. As noted earlier, actual, physiological basal needs are very low, and they are intermittent. As stated earlier, one does not need a consistent “drip” of insulin, so coming up with a ratio at all poses challenges.
Let’s start by considering the ideal case of normal weight T1Ds. Several studies explored this population, one of which is Ishibashi et al. 2021, J Diabetes Investigation. The investigators recruited lean T1Ds (mean BMI ~22 kg/m²), and put them under controlled hospital conditions with standardized diabetic meals and found their basal ratios were roughly 24.1% ± 9.8% of TDD.
A separate Japanese pump study (Kuroda et al. 2011, Diabetes Care), confirmed these figures, finding basal needs averaged ~27.7% of TDD.
Can it be that straightforward? That you just simply reduce your basal dose so it represents 20-30% of your “current” TDD?
Well, it’s a start. But keep in mind that these studies were not intended to be targets, but to show that lower basal ratios correlate to leaner individuals. Their A1c averaged 7.3%, which is not entirely ideal.
If your goal is to lose weight, then yes, reducing basal from 50% to 25% will help, and you could work to also lower your A1c levels. But remember—again—that actual, physiological basal needs are very low and intermittent, so your new 25% is still covering more than just background metabolic needs. Most basal dosing is covering meals, so you’re still going to get unexpected volatility and hypos. Potentially, just less extreme and proportionally more manageable.
Imperfect as it may sound, this is still going in the right direction, and the T1D universe would be much healthier at those ratios than 50/50.
So, why doesn’t the ADA recommend that? Short answers:
Lower basal now requires user attention to administer boluses “as needed” when glucose levels happen to rise if the liver produces glucose. This kind of attention is likely more than what many T1Ds are willing to tolerate. Many already feel overwhelmed.
For the ADA to make any changes to guidelines, they need to follow them up with very specific instructions for clinicians, all the way down the service provider chain: Nurses, practitioners, diabetes educators, etc. The diabetes services ecosystem is highly complex and interwoven. Making changes to guidelines is a massive undertaking.
So, for T1Ds who want to reduce basal dosing, it’s more likely going to be a solo effort. Under those conditions, the next question is how? Some may be thinking that they should be conservative by gradually reducing their basal dosing, little by little, thinking that it’s safer, or less disruptive.
The primary problem is that stepwise reduction adds new variables—bolus timing, food, activity—which quickly compound and make tracking nearly impossible. Each step reduction requires a new mental and physiological recalibration. It’s highly advisable that you do NOT attempt stepwise reduction. Pick a target ratio and just start there. If your target is 25%, start at 25%.
For purposes of discussion, let’s consider the logical endpoint: zero-basal dosing.
That may sound extreme, but as you’ll see later, it’s not only being done, it’s becoming more common. Remember, the only thing zero-basal dosing means is that you’re not necessarily looking to reduce your total insulin (though that could happen), it’s more that you’re shifting when you take insulin and what doses. In other words, the biggest change going to be your meal-time boluses, and then the occasional midday bolus “as needed” if your glucose levels rise. Surely, you can handle that.
Also remember, this is not necessarily recommended or, even if you do it, a permanent state of management. It’s purely an experiment to help you truly understand how much insulin you actually need, and when you need it. That’s very hard to do when there’s a background basal rate going, and you have no idea how much of it may be affecting meals or between-meal glucose levels.
You’ll quickly see that this is less dramatic than it first appears.
Zero-Basal Dosing
Zero-basal T1D management is not just possible, but it’s progressively becoming more mainstream. We’ll start with AID systems, and then move to MDI.
For those on automated insulin pumps, the DIY (do-it-yourself) community has been at the forefront of pushing the boundaries of insulin delivery, and their experiences and documentation show that the concept of an “emergent basal” – where the algorithm dynamically manages insulin from a very low or effectively zero baseline – is not just theoretical but actively explored and implemented by highly engaged patients.
Here are some references for DIY AID and Basal Strategies. Note that the term “SMB” refers to supermicrobolus, where the algorithm dynamically manages insulin using frequent, tiny insulin doses.
AndroidAPS alt-Guide. OpenAPS algorithm basal.
Details the fundamental principles of the OpenAPS algorithm and its inherent capacity to manage basal needs in a demand-driven, rather than strictly pre-programmed, fashion.
AndroidAPS-alt-Guide. Preferences (Max Basal IOB).
Advises new users to set the “Max Basal IOB” to zero initially, preventing the algorithm from adding any additional basal insulin beyond what’s actively needed. This allows users to observe how the system manages glucose through reductions and suspensions.
Diabettech. Understanding SMB and oref1.
An in-depth explanation of Supermicrobolus (SMB) and the oref1 algorithm, key components of many DIY AID systems. It describes how these advanced algorithms can deliver small, frequent boluses of insulin that can cover both mealtime and background (basal) needs, and how they safely implement temporary basal reductions, including to zero, to prevent lows.
Diabetes Canada. DIY AID User’s Guide - Clinical Practice Guidelines.
Recommendations for healthcare professionals engaging with individuals who use DIY AID systems. It acknowledges the high degree of self-management and continuous optimization undertaken by DIY users, including their ability to adjust basal rates, insulin sensitivity factors, and carbohydrate ratios to precisely match their physiological requirements. This resource validates the proactive, highly individualized approach to basal management found within the DIY community.
This is a fast-moving industry, and commercial pump manufacturers are following suit. For example, recent pump software upgrades allow you to set a temp basal rate of zero for many hours, including all night long (up from two hours in prior versions). There is strong real-world and preliminary clinical evidence that DIY users who employ zero basal via temp-basal suspensions—as integral parts of OpenAPS/AndroidAPS—achieve:
Higher TIR (~77–83%)
Lower average glucose (~137 mg/dL)
Improved A1c (~6.2–6.4%)
Reduced hypoglycemia (~2–4%)
These metrics are comparable or superior to results from commercial AID systems, suggesting that on-demand basal suppression not only works safely but outperforms traditional basal-based strategies.
Engagement is key
The main caveat here, which continues to be emphasized, is that this group of people are highly engaged with their T1D. They are tech-savvy, typically younger, super-educated, and highly motivated. A classic case of selection bias.
Automated systems definitely work, just not this well among the general population. As I detail in my article, Medical Literature Analysis: The Performance Paradox of AID Systems, population-wide data from medical literature shows that the greatest beneficiaries are those who are entirely engaged, or are entirely disengaged.
While that may sound counterintuitive, it’s because the “success” of an AID system is measured by A1c improvement relative to before they started on automation.
For example, if a person’s A1c is >9%, and their TIR is <50%, they will see a dramatic improvement, sometimes up to ~70% TIR and an A1c of 7%. This group includes children, those with mental disorders, and those who cannot or will not engage with their disease. For this subset of T1Ds, AID systems are amazing. It’s not surprising that this is where the headlines are. Indeed, every T1D conference I go to talks glowingly about a child whose TIR is <20%, suddenly going to 70% on an AID system, and the crowd gasps and applauds.
Similarly, AID systems give highly engaged users benefit, because it allows them to go from 7.5% to 7%, or even better in outlier cases, largely because they are able to go to zero-basal dosing. Again, this is where the headlines are.
But the vast majority of T1Ds lie outside of these two endpoints, largely because most are at least partially engaged—they remember to bolus for meals, and have a pretty good idea of how much to bolus under varying conditions. So, they’re in the middle of the engagement spectrum. This group is unlikely to see much benefit from an AID system.
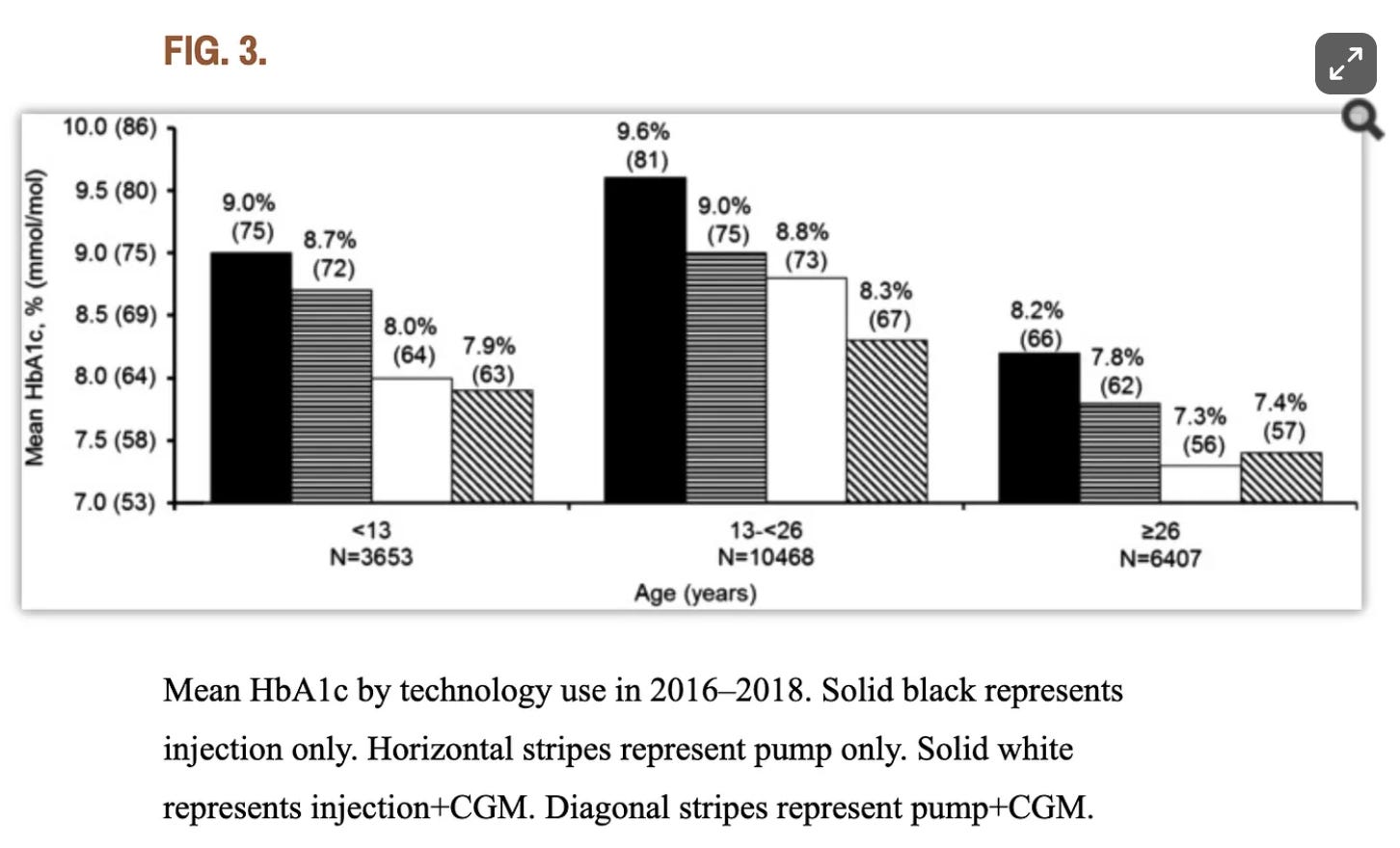
And despite the fact that AID algorithms continue to “improve” (purportedly), and the uptake has also increased, population-wide data continues to show that AID and MDI users achieve largely identical outcomes since at least 2018. That year, a research study by the T1D exchange found that, for adults over 26, “there isn’t a substantial difference between A1c outcomes for those who manually take insulin injections compared to those who use pumps,” including closed-loop insulin pumps. Below is a figure from that study. (The age group on the far right are those >26.)
This is not a critique of AID systems by any means! Well, there is one critique of AID systems: The attribution of causality. T1D outcomes are not due to technology, it’s due to user engagement. For zero-based basal dosing to work—or any level of basal adjustment, even just 25%—you have to be engaged.
Even the pump companies themselves will say this: They can’t do as well by guessing—even with their advanced algorithms—than if you engage with the system. In Medtronic’s documentation for the MiniMed 780G system, they state:
“Carb counting is an essential component of diabetes management … and is still very important in automatic insulin delivery systems as per system design.”
The best way to learn how to be engaged is, ironically, to be engaged.
It’s a learning process that’s similar to anything else in life—a sport, a musical instrument, using new software, or learning a new language. It’s not so much about memorization; it’s about the experience of learning. Experience is the best teacher. As you get more familiar with the intricacies of your pursuit, the intensity of early adaptation lifts and your own autopilot kicks in, and it becomes second nature.
When you watch an AID system do its thing, you learn from it, but—here’s the catch—you also see its deficits. You catch it when it fails to dose an amount you think it should. You reset targets when you know it should. As long as you remain engaged, you do better. If you’re entirely disengaged, it’ll do its thing, and while the outcome may be better than what you can do on your own, it won’t be “healthy” insofar as long-term complications and mortality risk.
Using an AID system is not the only way to stay engaged. MDI users are also engaged, if not more so, simply because they have no choice—there’s no “automation” they can outsource their management to.
I’ll share my own experience with this, and as you’ll see, I’m MDI.
The Active Learning Boot Camp
In 2020, I grew increasingly frustrated with hypoglycemia, especially at night. I wanted to finally nail down this problem. And that’s when I remembered something I had used when I taught entrepreneurship at the University of California Santa Cruz: A pedagogical model called active learning. It refers to instructional methods that directly engage students in the learning process through activities like problem solving, discussion, case studies, and experimentation. Instead of passively receiving information (e.g., lectures or substack articles, cough cough), students construct knowledge actively through doing, questioning, and immediate feedback.
I figured I should try this on myself. To truly deconstruct why my hypos were too frequent and unexplained, I decided to engage in an experiment: Dedicate two weeks to paying really close attention to my CGM—virtually glued to it—watching for glucose patterns and pairing them with insulin doses and carb intake.
I call it the Active Learning T1D Boot Camp. I wanted to start afresh. Throw away the rulebook, and just experiment using the most basic core principles I knew: Dose for meals and when glucose levels rise, and nudge the wheel this way and that as necessary throughout the day.
Active learning in T1D isn’t just mechanical. It builds metabolic intuition. Much like a student internalizes algebraic thinking or musical phrasing, a T1D actively builds a mental model of when and how much to pre-bolus for certain meals, how to dose for fat+protein, how long post-exercise glycemic dips can last, which time of day insulin is more or less effective.
This accumulated experience functions like a personalized metabolic curriculum, crafted and mastered through daily iteration. As with any learning, once you get it, you know it—it’s not work anymore. It’s now your expertise.
So, I got an InPen, a bluetooth-enabled insulin pen, and downloaded Sugarmate, a T1D management app, so I could see realtime glucose patterns and insulin dosing. I then started my two weeks of intensely watching my CGM. I literally watched it every 10-15 minutes, fascinated by how my glucose levels would move to different kinds of foods, insulin dosing, where I took insulin (leg vs. arm vs. abdomen). Or even just watching TV.
I mounted my phone next to my computer so it was always in view. I mounted it in my car—same thing. I glanced at it while hiking, running and walking. It was always on, and I was amazed at how differently I interpreted glucose changes while watching in real time versus just looking at a day-long or week-long chart/analysis after the fact. It’s a whole new world.
Then came the unexpected: Because I was always watching, I was proactively making frequent interventions. Sugar up after a burrito? Dose! Sugar down after exercise, eat! Don’t wait—do it now, when you see it.
By paying attention all the time—and reacting in real time, not delayed—I was able to correlate actions with outcomes. This wasn’t just factual. It was experiential. Each new pattern and cause-effect outcome was mentally noted. There would be variations, but with enough repetition, you see the patterns within the patterns.
And it was only two weeks long. And during that time, my TIR rose from 70% to well over 85%. In fact, I saw many days at 100%.
Here’s the shocker: I did not necessarily discover why I was getting so many hypos, especially at night. Sure, I could intervene in a timely manner, but why they happened with the same severity and frequency remained a mystery.
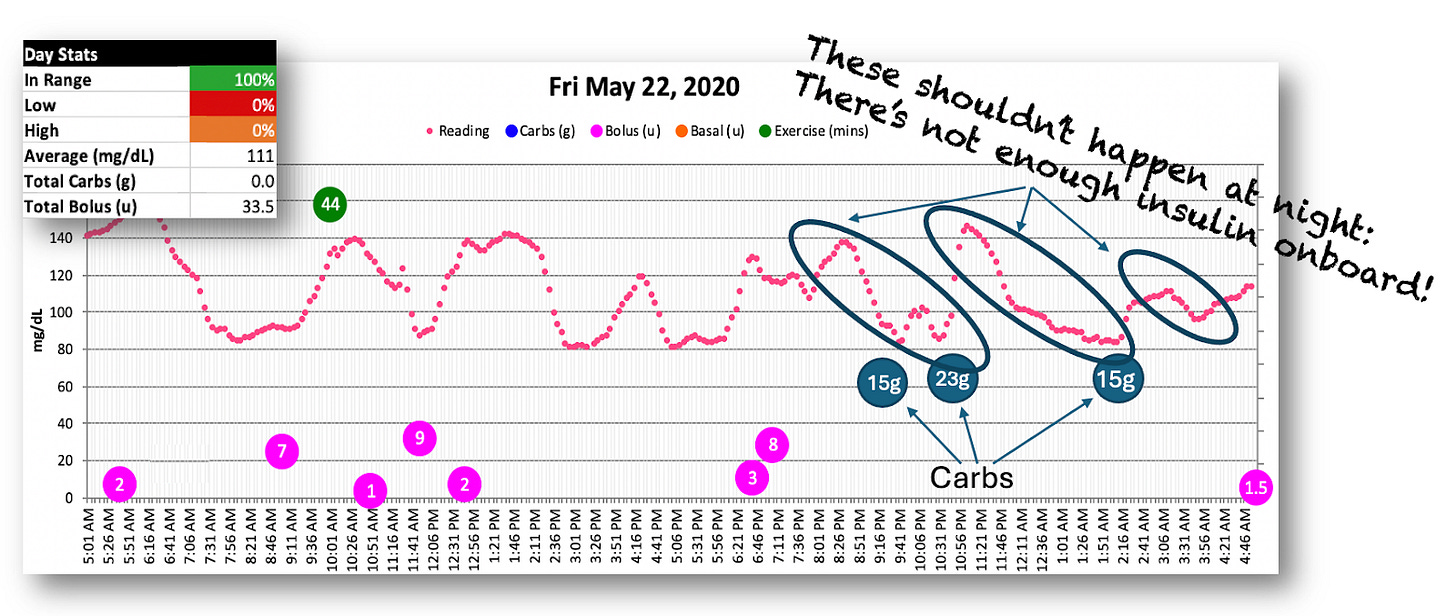
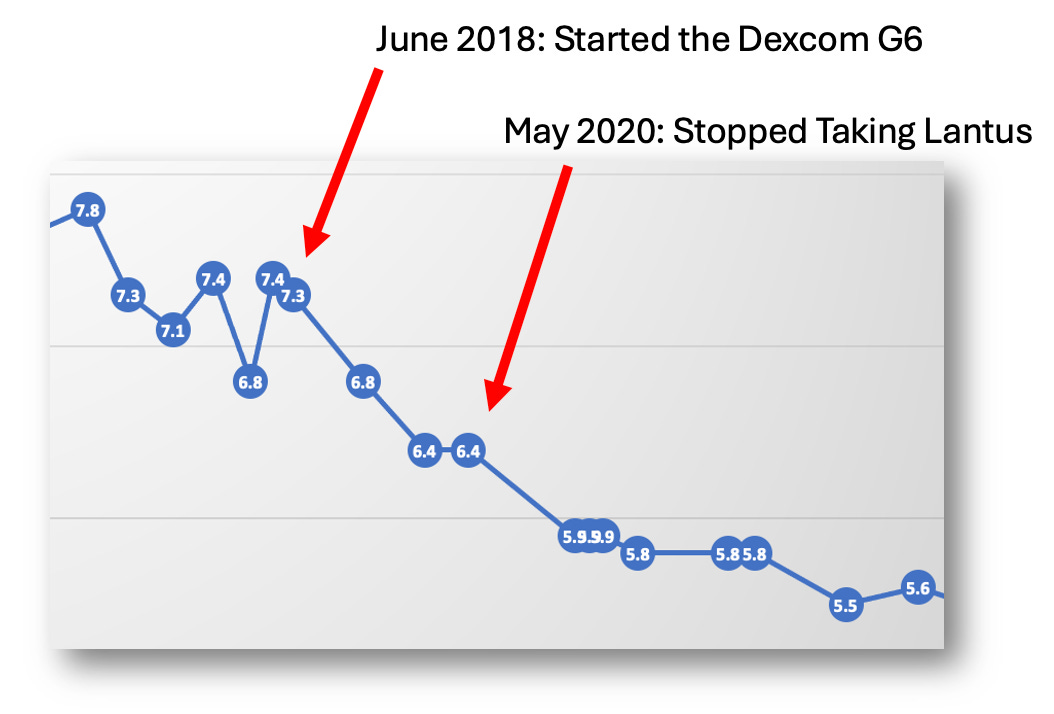
Below is a chart of a typical day in May 2020 that illustrates this. (Note: I didn’t log carbs on a regular basis yet. The three night time notations in the graph were added separately from the software.)
Yes, I was 100% in range, but the volatility is pretty extreme as measured by the frequency of the peaks and valleys and the number of interventions I needed to do to stay in range.
But you can also see that the night time hypos were still going strong. Despite no bolus dosing after 7pm (ish), I was still having to eat tons of dextrose tabs just to stay in range all night long.
Lantus: The Stealthy, Evil Agitator
Staring at this chart, it dawned on me: I could see all my boluses, but I did not see my Lantus doses because I didn’t log them. Lantus is supposed to be “background” insulin. Just set it and forget it, right? So why log it?
That’s what gave me the epiphany: Lantus must be responsible for both the volatility and the hypos!
When I told my Endo this, he didn’t suggest reducing the basal dose. Why? Because it was generally understood that T1Ds need ongoing basal insulin day and night. You know, the 50/50 rule. And my 10u/day wasn’t even there. It was more like 30% of my TDD, far lower than ADA guidelines.
But one thing he did say that got my attention: “You might try splitting your Lantus dosing: Do 5u in the morning, and 5u before bed. Some people say the absorption curve is better.”
Hold on there: Splitting the dose? I thought Lantus was supposed to have a relatively “flat” absorption curve. If splitting 10u Lantus into two doses is better, how about three? Four? How far can we dice this up? Theoretically, the more you dice it up, the “flatter” it should be, right? After all, that’s what we’re after, right? Flat!
As I would later learn, flat is exactly what’s causing the problem. A human body does not want a steady, constant, continual drip of insulin all the time. It wants it only when needed, in the amount that’s needed, and that amount is very, very low.
I didn’t know any of that in 2020. But this “dose splitting” idea inspired my next experiment: If I really wanted to figure this out—how much “background insulin” I truly needed, and when—I needed to stop taking Lantus cold turkey, and just bolus with Humalog “as needed”. If I figure out the timing and the amount, I could resume my Lantus more “intelligently”.
What made me nervous was the night, but I decided to do it anyway. If I needed to wake up and take insulin, so be it. I already have to wake up all the time to eat glucose tabs.
So, in late-June 2020, I stopped Lantus cold turkey.
Basal Insulin Getting Kicked Off the Island
After I stopped taking Lantus, the first thing I noticed was the hypos virtually disappeared. Not completely, but mostly.
But here’s the other thing I noticed: There was no basal curve. I hardly did anything differently. I went from intensely focused on my CGM and making frequent interventions to just…watching. My daily management was far easier. My volatility (measured by “standard deviation”) was far lower.
In short, Lantus added no benefit at all—ever. For me, taking basal insulin did nothing more than put more insulin into my system that I just didn’t need. And that was causing the hypos, forcing me to constantly compensate with carbs, which had accounted for why I was always a tad more plump than I wanted to be.
I know what you’re wondering: What about NIGHT?
Ah yes, that mystery.
Nocturnal Hypoglycemia
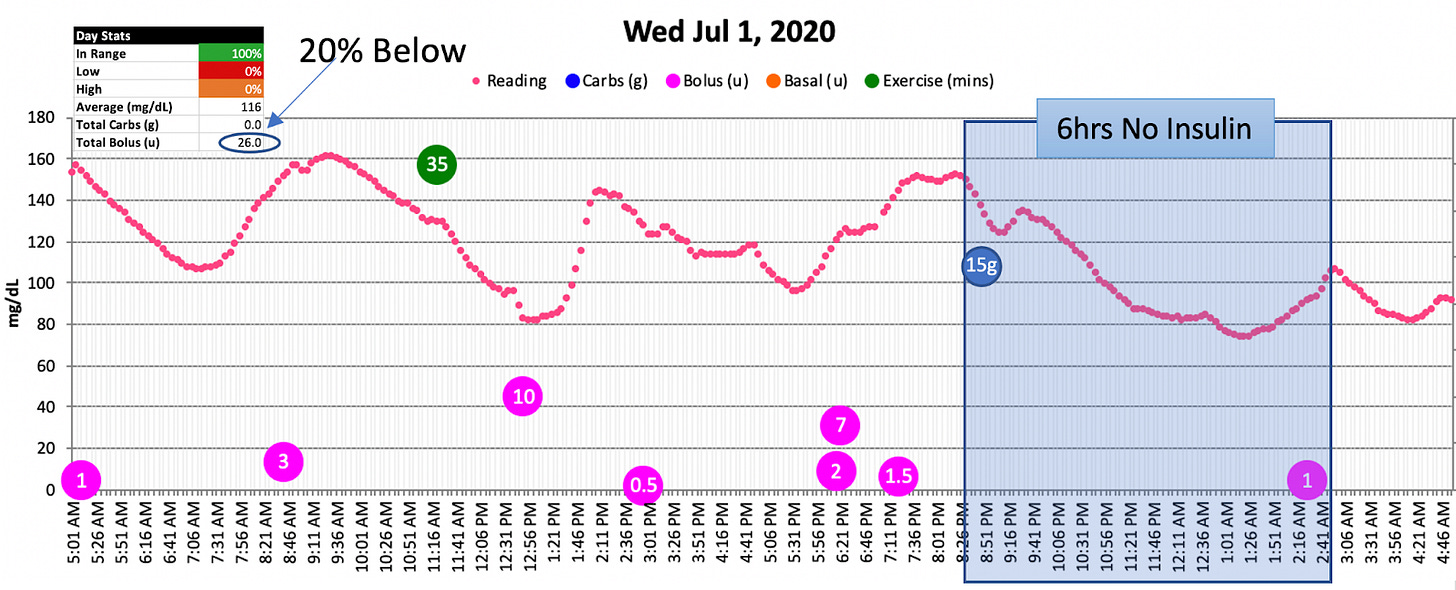
See if you can spot it in this chart:
Yes, my glucose levels dropped and night, despite not taking any basal insulin, or boluses before bed! Indeed, this blue box is an insulin-free gap. And yet, my glucose still dropped. Many of those gaps would last anywhere from 5 hours to up to 12 hours straight! Curious, right? Here’s another illustration.
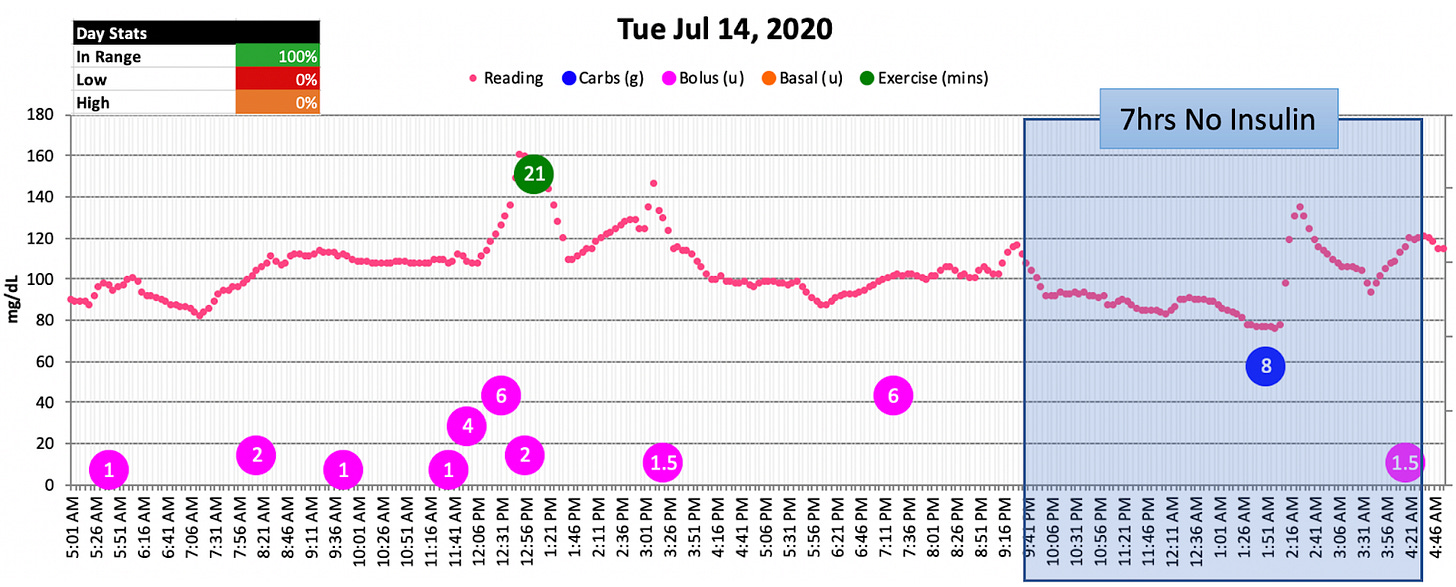
This chart represented my new normal: Every single night had insulin-free gaps. And even then, I still had mild hypo events. I would always have to eat carbs to compensate for the hypos. And after a quick spike, my glucose levels would drop right back down.
So, what explains the hypos at night, despite no insulin onboard?
Non-insulin mediated glucose uptake—or NIIMGU. As you no doubt recall in the third article that I keep reminding you to read, a lot of blood glucose is taken up by tissues that do not need insulin mediation. In fact, as the article explains, 75% of glucose in the body is taken up this way, leaving only 25% of tissues needing insulin to take up glucose. This is particularly evident at night, where NIMGU processes pull far more glucose from your bloodstream than other times of the day.
Remember, “hypoglycemia” is about the deficit of glucose, not necessarily an excess of insulin. This is why my hypoglycemia was more severe when I was taking Lantus: Both NIMGU and Lantus were pulling glucose out of my bloodstream all night long. Hence, severe hypos. Without Lantus, I’d still have hypos at night due to NIMGU processes, but they were fewer and less severe.
Naturally, this feels highly unusual. Few people have ever heard of it, right?
You have, you just didn’t know it.
According to a literature review in Science Direct, the primary cause for nocturnal hypoglycemia is basal insulin dosing, where prevalence rises for those who exercise.
It’s far more common than people think. According to a published analysis in the journal, Diabetes Care, nocturnal hypoglycemia occurs on approximately 8.5% of nights (ranging from 3.7-12.1%), and most events last for 2 hours or longer. Among patients under age 40 who died over a 10-year period, 6% of deaths were attributed to “dead-in-bed” syndrome, which is often the result of severe nocturnal hypoglycemia. In a separate study that lasted only 4 weeks, 54% of T1D patients experienced at least one nocturnal hypoglycemic event.
This is exactly why closed-loop systems allow you to set zero-basal profiles for night time.
The reason my situation appears more extreme is high intensity exercise, which depletes muscle glycogen stores. The higher intensity, duration and frequency, the greater that depletion. Here’s the kicker: Glycogen stores are replenished overnight via NIMGU. The more depleted they are, the more glucose is needed in the bloodstream. If there’s insufficient glucose in the bloodstream, you go hypo, even if you have no insulin onboard. This is a well-documented phenomenon called Post-exercise latent-onset hypoglycemia (PELOH).
This is a significant issue for those on low-carb diets because the alpha cells will not signal the liver to produce glucose, and even if they could, the liver uses its own glycogen stores to make that glucose. And the only way to fill glycogen stores is by consuming glucose or other kinds of simple carbohydrates. Low-carb diets can still allow a person to do aerobic exercise because they can oxidize fats that break down into glucose, but fat oxidation cannot be used to replenish glycogen stores. For more, see my article, The Paradox of Low-Carb Diets: A1c vs. Metabolic Health.
Another point that should be noted is that insulin-free gaps do not raise the risk of DKA. For more, see my article, Ketones: The Unjustly Demonized Villain in T1D Management, which explains the metabolic benefits of ketones, and how the unfounded fear of DKA is partially responsible for high basal dosing guidelines.
The lesson about nocturnal hypos is important: Night time basal dosing should be considerably lower than conventional wisdom. Few will be “insulin-free” as I tend to be, but your mileage may vary.
For MDI users, a light dose of a conventional basal insulin should do, but some even use plain old Regular insulin that’s been around for decades. It has an eight hour lifespan, though mileage varies. Indeed, all insulins vary considerably from one person to the next. It just requires experimentation. Also, there’s a lot of variability. You may find that 1u more or less may yield different results, but this would be the case if you made no changes.
Sleep quality and duration have a huge effect on cortisol, which can spike under physiological stress. That could often double your basal needs at night, and it certainly has a huge effect on the dawn effect as well.
Do not expect consistency from night time basal dosing. It’s expected that you’d need a correction unit here or there, or perhaps even a dextrose tab or two as well.
Post-Boot Camp Debrief
By terminating Lantus and understanding my metabolic profile, my TIR went up from 85% to 95%, and my A1c dropped to 5.5%. Oh, and I lost 15 lbs.
Best of all, my “daily management” was far easier, less intense, and less risky. I didn’t fear hypos anymore, and was more confident in my eating and exercise.
Sure, I still get huge spikes and unexpected lows—that’s T1D for ya.
Best of all, once I learned my metabolic profile and how to read patterns, the intensity of daily management eased off. I’ve never been obsessed with numbers or have anxiety about being “in range” or other concerns. My motivation was solely driven by a desire to reduce hypos. Everything else was the proverbial icing on the cake. Or, the silver lining. Pick your aphorism.
My experience is unlikely to be like anyone else’s, but yours won’t be like others’ either. All T1Ds are very unique from one another, despite what may appear to be some superficial similarities. For example, we all take insulin. Outside of that, you have to learn your own profile to achieve healthier outcomes. The most important phase in the active learning process is the first two weeks: the technique. Once you get it, you are equipped throughout life.
Active learning is not a way out of T1D management. It’s a way through it.
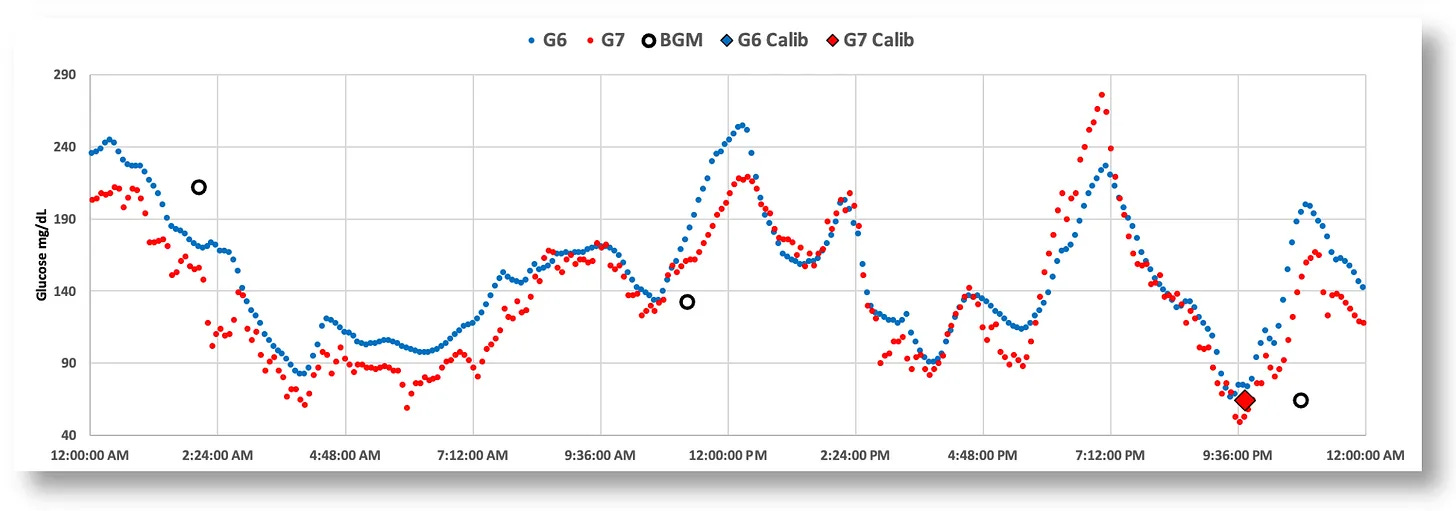
Lastly, it needs to be stated that which CGM you use matters a lot. Not all CGMs are equally suited for this approach. As I detailed in my article, Continuous Glucose Monitors: Does Better Accuracy Mean Better Glycemic Control?, there are significant differences between CGMs in sensor noise and reliability for in-the-moment pattern analysis. At a high level (day-long patterns), any CGM will tell you TIR and other macro-level data. But active engagement requires better precision. As a teaser, here’s a screenshot from that article that illustrates the difference between the Dexcom G6 and G7.
As my article details, the Dexcom G6 consistently demonstrates lower noise and more stable readings, making it particularly well-suited for the precise, proactive management that active learning requires, whether you use an AID system or MDI.
Final Words
Remember, my goal was not to aim for a particular A1c or TIR target. My goal was to reduce those awful hypos. It turned out that high basal dosing triggers hypoglycemia during and after exercise. In fact, fear of hypos is the primary reason why T1Ds don’t exercise, and the first step in avoiding this problem is by severely reducing basal dosing. I’m glad my fear of hypos didn’t discourage me from exercise (as it does for most T1Ds), or I’d be much worse off.
Indeed, metabolic health—which only exercise can provide—is far more determinative of T1D complications and lifespan than even A1c levels. For more, see my article, T1D and Health: How Long Will You Live?, where I cite long-term risk factors and their correlation with A1c, metabolic health, and cardiovascular fitness.
As the research shows from that article, someone with an A1c of 5.5% and poor metabolic health will likely die sooner than someone with an A1c of 7% and excellent metabolic health. It just so happens that modern techniques now allow for the best of both worlds. Exercise and low basal dosing combine to yield both better metabolic health and better glycemic control.
My next article in this series gets into exercise and T1D physiology, so stay tuned for that.
If you would like to try the active learning boot camp, but have reservations, see my article, The Four Habits of Healthy T1Ds, as it talks about the science and psychology of habit-building.



Never even heard of the 50/50 thing until relatively recently (dx'd in 1983). I started on a pump while under care of Joslin and they just had me do the usual fasting/testing thing (pre-CGM). Over time I've tweaked my settings when fasting levels indicated the need, and of course now I use an AID pump (Tandem CIQ). Thing is, I eat restricted carb, so my basal / bolus ratio leans much more toward basal just b/c I don't bolus all that much. Currently I'm at ~70/30 basal to bolus, with a 5.3 AIC and 95-100% TIR. I don't see how I'm going to benefit from trying to drop my basal component to a lower percentage than bolus under those conditions.
Hi Dan, I'm curious about the tradeoff between basal and sleep quality. As other commenters have pointed out, and as your article shows as well, you take zero basal units but either still experience overnight hypos OR pre-dose carbs to avoid it. Both of these would seem to impact sleep quality - you're waking up to take glucose or you're waking up to dose to come down after over-eating. Even if you successfully pre-carb and don't have to wake up, the digestion process is impacting your sleep as well, right?
My question is: given what we know about how important sleep quality is for long-term health and reduction in all-cause mortality, is this tradeoff worth it? E.g. if you were on a 30% basal ratio and generally experienced no overnight interruptions, would you still try to reduce basal to zero knowing that it would likely increase the amount of overnight interruptions and thus reduce sleep quality overall?