Insulin and Heart Disease: What You Should Know
Insulin and glucose are both essential to live. Too much of either can be harmful.
Most people know that elevated glucose levels is an indicator of diabetes, which can lead to all sorts of disorders, especially cardiovascular disease (heart attacks and strokes).
If you become diabetic for whatever reason (there are eleven different types) you quickly learn that insulin lowers glucose levels.
It would seem natural to assume, therefore, that you should take whatever amount of insulin is necessary to keep glucose levels lower. You know, to reduce cardiovascular risk.
But, what if insulin itself is actually doing similar—or potentially worse—harm to your vasculature than elevated glucose levels?
This presents a very challenging problem, of course. But if you’re suspicious of this notion, let’s backtrack a bit.
The association between too much insulin—hyperinsulinemia—and cardiovascular risk has been known as early as two years after insulin itself was discovered in 1929. According to a literature review article, “Insulin Resistance and Atherosclerosis : Common Roots for Two Common Diseases?”, cardiologist Paul Dudley White noted in 1931 that “diabetes favors arteriosclerosis” and that an “excess of insulin” might contribute to this condition.
In the 1996 article, “Insulin resistance and atherosclerosis,” the authors highlight Gerald Reaven's 1988 Banting Lecture that introduced “Syndrome X” (now known as metabolic syndrome), highlighting the role of hyperinsulinemia in atherosclerosis.
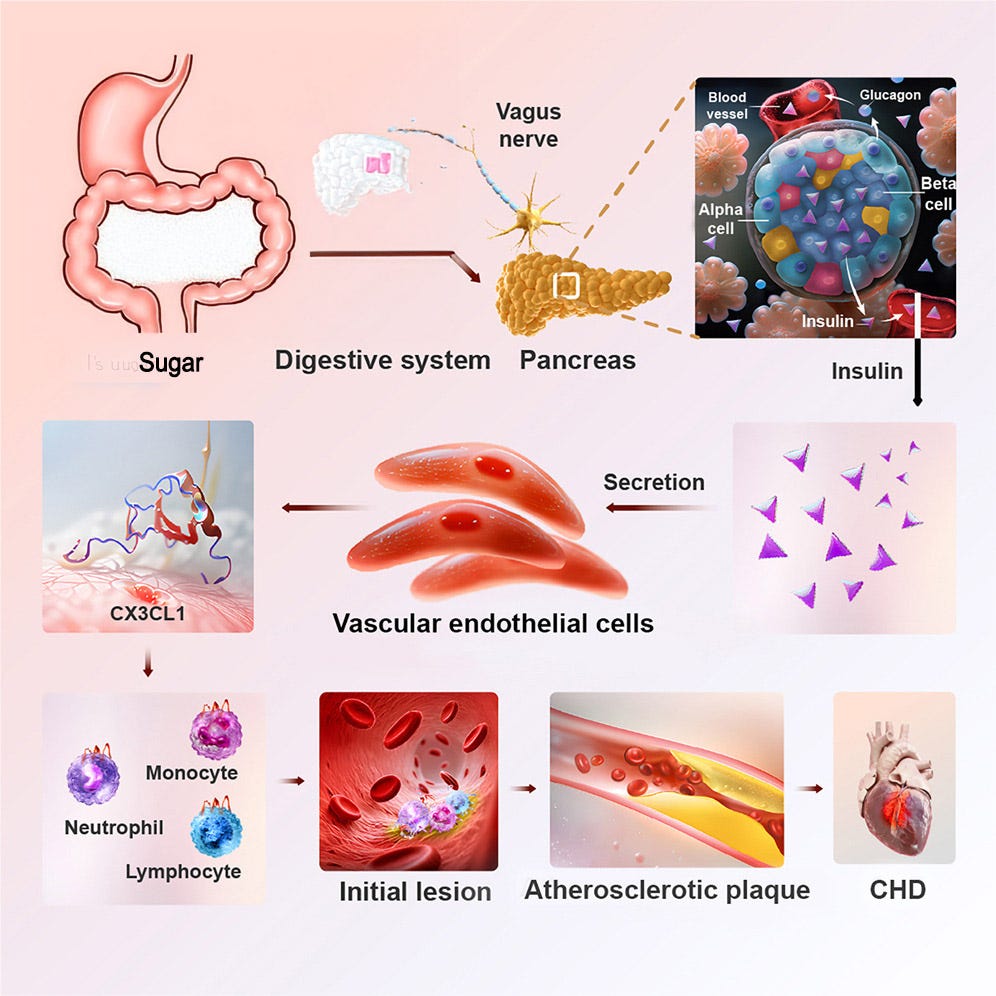
Not only has this been known for so long, the actual cellular mechanisms by which it happens has also long been known. The illustration below shows how this pathway works: Sugar enters the digestive system, which signals the beta cells in the pancreas to produce insulin, which enters the vascular endothelial cells, which then trigger the CX3CL1 pathway that leads to an immune response, which creates a lesion that leads to plaque (calcification of the arteries).
Of course, the amount of insulin needed to produce harmful levels of plaque is high, which usually takes decades of elevated insulin levels. And that’s how and why researchers discovered the association between elevated insulin levels and cardiovascular disease in both type 1 and type 2 diabetics: Both are exposed to huge amounts of insulin over the course of their lives as they aim to reduce glucose levels. In T2Ds, the beta cells produce excess insulin to deal with the overconsumption of foods, and in T1Ds, the person manually administers excess insulin.
Despite the fact that this has been known for decades, never is it ever discussed between doctors and patients. Instead, people are so focused on lowering glucose levels—which is clearly very important, make no mistake about it—they lose sight of this other known risk posed by insulin.
To illustrate just how little known (or considered) this phenomenon is, let’s go back to the whole idea about glucose control, and for that, we bring in one of the oldest—and wrongly accused—villains: aspartame.
Aspartame: The wrongly accused villain
As all diabetics know, artificial sweeteners are a helpful tool for reducing calories and stabilizing blood sugar. Yet despite decades of research showing their safety, these sweeteners frequently find themselves in the crosshairs of sensational headlines, and these, in turn, are affecting diabetics’ dietary choices.
It would be exhausting to run through them all, but a recent paper published in Cell Metabolism is a good candidate for understanding how things go wrong. While we’re at it, we can learn some good things too, like who the real villain is.
The paper we’re going to be looking at is called, “Aspartame Exacerbates Atherosclerosis through Insulin-Triggered Inflammation”.
Most people reading that title stop at the first three words — "Aspartame Exacerbates Atherosclerosis" — and they panic. That's all they need to see, and it sets off a cortisol reaction that itself leads to atherosclerosis.
Let's not get ahead of ourselves. This study — including both its valid findings and its flaws — can teach us some important lessons about both health and science, especially for diabetics.
Why the Authors Studied Aspartame
The study authors set out to investigate how aspartame might influence heart disease risk. That is, they wanted to study the “mechanism of action.” This is important because there are lots of observable associations between events and detrimental harm, but that doesn’t mean we understand the cellular mechanisms involved. For example, we know the harmful effects of smoking cigarettes, but there’s never been a way to describe the actual cellular mechanisms that lead to things like cancer.
As this pertains to the headlines associating harms of sugar substitutes with cardiovascular disease, one would think finding this cellular connection would be a useful scientific inquiry. (Of course, this will only work if there actually is such an association. Keep that in mind.)
To do this, the researchers started by feeding mice both aspartame and sucrose (table sugar) to measure the amount of insulin secreted by each substance. The purpose was to establish a well-known fact that both aspartame and sucrose increase insulin secretion. Nothing new.
Wait, you’re wondering. Why bother to look to see if aspartame increases insulin levels? Isn’t aspartame the bad chemical here? Isn’t it doing harm to arteries?
Ok, hold on. Didn’t we just review the problem with elevated insulin levels? Did you already forget? Of course not, but the researchers in this paper certainly did. Instead, they wanted to blame aspartame, for reasons we can’t infer, but as we deconstruct the actual steps taken in their study, it’s plainly evident this was their predetermined goal.
But, first, let’s address what’s clearly on your mind anyway: Aspartame has been attributed to all sorts of problems, so why not look into it?
Ok, let’s do that.
Aspartame is not some synthetically manufactured chemical running around your body causing cancer or inflammation. It’s just a very simple molecule composed of two amino acids and methanol (a type of alcohol) all of which are found naturally in many fruits and vegetables. In fact, 1 liter of diet soda provides ~55 mg of methanol, whereas 1 liter of tomato juice provides ~200 mg of methanol.
Aspartame is not absorbed into the bloodstream; upon ingestion, it is completely hydrolyzed in the small intestine by digestive enzymes. None of these accumulate in the body.
In fact, you could drink dihydrogen monoxide (DHMO) and get roughly the same effect, more or less.
Aspartame got its bad rap from several alarming studies that involved outrageously unrealistic doses — equivalent to drinking 200 cans of diet soda every day for years. Follow-up studies have consistently debunked this idea when using realistic human consumption levels. Major regulatory bodies, including the FDA, EFSA, and WHO, have repeatedly concluded that aspartame is safe.
Aspartame just sounds like an evil chemical, but it’s really not. Hence, the comparison to dihydrogen monoxide, which, shockingly, is simply water (H₂O). See? That’s not so bad, is it? Tons of surveys have found that people are highly afraid of dihydrogen monoxide, simply because they don’t know what it is, and it sounds “chemical-y.”
But that doesn’t mean dihydrogen monoxide can’t kill you. Drink too much of it and you’d get water intoxication or hyponatremia and DIE!
Bad right? But no one talks about the dangers of drinking too much water. So, whenever there’s a study investigating something, and they say they see effects if you drink 200 cans of it, even dihydrogen monoxide, it’s best to be skeptical.
That’s the fun of chemistry: It can make anything sound evil. You know, like “Liquid Death,” a brand of tap water sold in cans designed frightening-looking artwork.
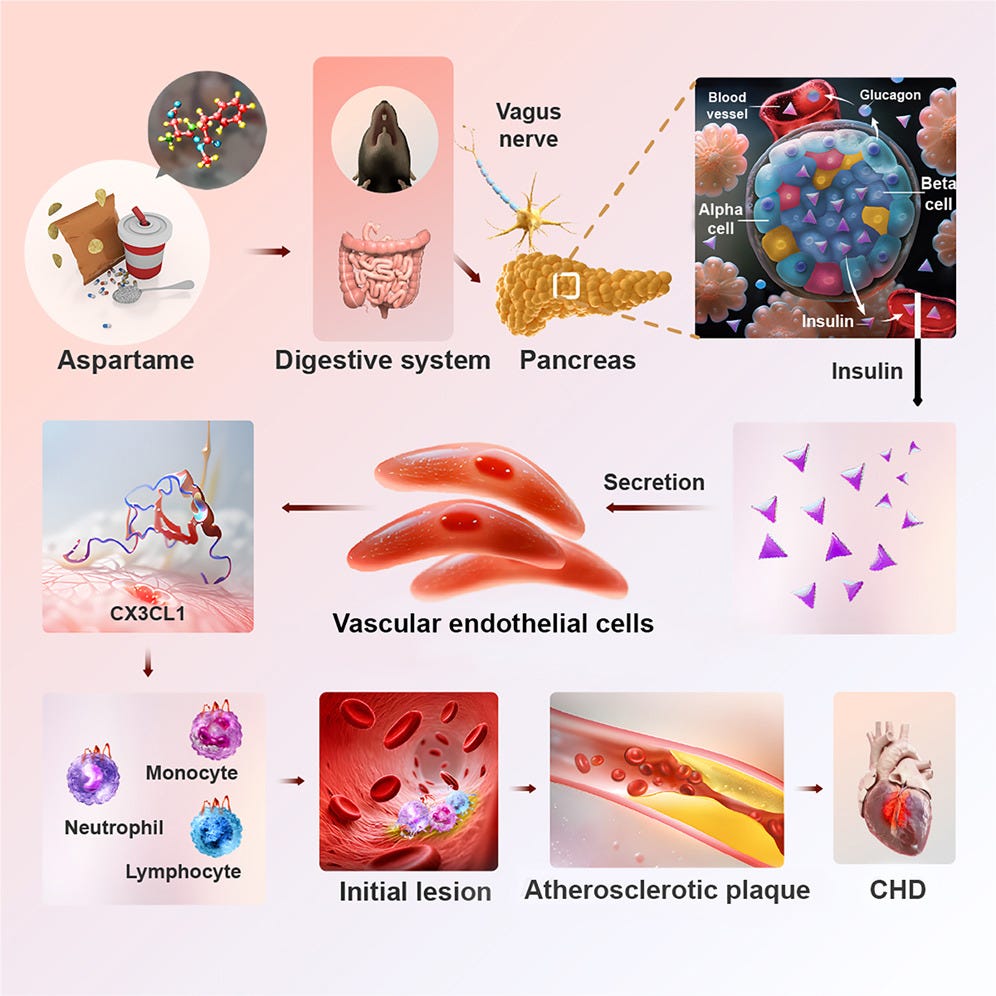
But, unlike water, aspartame increases insulin secretion, despite the fact that it doesn’t raise glucose levels. The following illustration comes from the paper, showing that the researchers fed mice aspartame, and they showed something that will look very familiar.
The researchers merely showed that aspartame triggers an insulin response, through exactly the same mechanisms that sugar does.
Aspartame is no more unhealthy than sugar, insulin, or dihydrogen monoxide. In reasonable amounts, it’s fine. Too much, and you die. This is true of literally everything, including those things you eat that are “healthy.” Anything can be toxic if the dose is too high.
This is called biphasic — a pattern where low to moderate levels of something may be healthy, or even essential for life, but elevated levels can be harmful, toxic, and lead to death.
Insulin is no exception. If you take too much for prolonged periods, it can trigger inflammation, leading to an immune response, which leads to atherosclerosis. This is why heart disease is the leading cause of death in America: Too much food causes the beta cells to produce increasingly more insulin (hyperinsulinemia). As insulin levels rise, it causes inflammation that leads to insulin resistance, which creates a vicious cycle where the body just churns out more and more insulin to get past the resistance so it can lower glucose levels.
This is called Type 2 Diabetes, and while all this is happening, atherosclerosis builds up, leading to heart attacks and strokes. According to a study published in the Lancet, over ¾ of Americans are overweight, while the number of people with type 2 diabetes (including pre-diabetes) has doubled (since 1990) to well over 150 million.
If you’re a T1D, you probably already know we have no working beta cells, so aspartame isn’t going to cause insulin levels to rise, so the substance has no effect here. You know, like dihydrogen monoxide.
But, you can still get too much insulin, not from beta cells, but by injecting it. In T1Ds’ efforts to chase down glucose levels, many are taking too much insulin, creating the same conditions as T2Ds. Hence, the term, “double diabetes.”
In an interview, Dr. Ralph DeFronzo, a leading expert in diabetes research, noted that a healthy, lean T1D typically requires between 35-40 units of insulin per day to maintain normal glucose control. Once you exceed that level, it leads to tissues developing resistance to it, and that resistance causes inflammation, the hallmarks of T2D. In fact, the rate of obesity among T1Ds reached 37% in 2023 compared to only 3.4% in 1986, according to the Lancet article, “Obesity in people living with type 1 diabetes.”
See how it’s all starting to come around? So, in the present study, the researchers are looking to see how much aspartame is needed to increase insulin levels to a point where it’s atherosclerotic. Again, if aspartame only increased insulin at low levels, that’d be ok. But if it raises insulin levels too high, it can induce “insulin-triggered inflammation.”
All this leads us back to the title of the Cell article, “Aspartame Exacerbates Atherosclerosis through Insulin-Triggered Inflammation.”
Note that it doesn’t say aspartame causes atherosclerosis. It says it exacerbates it, and it does so via insulin-triggered inflammation.
Therefore, the key to understanding this particular study is to scrutinize how they conducted the study, whether their methods were reasonable, and whether their conclusions were credible. And this is where things unravel pretty quickly.
Unrealistic Dosing and Design Flaws
The researchers began their study by giving mice an aspartame dose equivalent to a human consuming roughly three cans of diet soda per day. At this dose, the researchers saw no significant changes in insulin secretion, inflammatory markers, or any signs of cardiovascular risk. Note that they also used sucrose to stimulate insulin, so the side-by-side comparison showed equivalency.
They then escalated the aspartame dose incrementally, little by little, inch by inch, until they saw those inflammatory markers. By the time they saw this effect, the aspartame levels were equivalent to drinking 200 cans of diet soda per day. (Where have we seen that number before?)
Ok, they saw an effect, and they could have published, “Drinking 200 cans of Diet Coke per day can lead to atherosclerosis.” But that’s not very interesting or useful.
And then, for reasons not explained in the paper—beyond what anyone else has been able to figure out—the researchers also put the mice on insulin pumps, not unlike the kind T1Ds use. Yes, they added more insulin to the poor little mice.
Imagine putting a pump on a non-diabetic and watching what happens to them. Not pretty. This step doesn’t seem to make any sense, and the paper doesn’t explain why they did it. But if they wanted to increase insulin levels to trigger the known inflammatory pathway that we already know about, then this is a good way to do it.
Lastly, and this is important, but also not clear as to why they did it: The researchers selected genetically modified mice that were already predisposed to atherosclerosis. As Oliver Jones, a professor of chemistry at RMIT University, noted in his blistering critique of this study: "A mouse model that is genetically engineered to be more susceptible to atherosclerosis is hardly representative of a typical human."
What an understatement.
That, plus the fact that they attached insulin pumps to the little guys and gave them 200 cans worth of diet coke everyday… No wonder they got atherosclerosis. I bet they also got a case of bad vibes.
And even then there’s not much to learn here. Unlike mice, humans don’t respond to aspartame the same way. According to a systematic review of randomized trials involving real people and aspartame, titled, “Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review of randomized controlled trials”, aspartame's components stimulate receptors in the gut, which is relayed through the vagus nerve to the pancreas, which may or may not signal beta cells to produce insulin. In humans, this is a very slow and weak response, but also highly variable among individuals—and often absent altogether!
By contrast, mice have far more sweet taste receptors (T1R2/T1R3) in their gastrointestinal tract, a more sensitive and responsive vagus nerve in relation to digestive signaling, and a gut-brain-pancreas communication loop that’s far more efficient at relaying signals. All told, mice insulin secretion is considerably higher, and therefore, non-comparable to humans.
One thing that mice and humans do have in common though: our ability to metabolize dihydrogen monoxide.
Why Did This Study Get Published?
Studies like this — with questionable design choices yet published in high-impact journals — aren’t rare. Understanding how this occurs can help readers better evaluate scientific claims. Let’s begin with the genesis of how and why the researchers set about doing this study in the first place from this article in Medical X Press, which interviewed the researchers:
The research was inspired by a can of diet soda during a project meeting. "One of my students was sipping on this sugar-free drink, and I said, 'Why don't you look into that?'" recalls senior author Yihai Cao. Previous research has linked consumption of sugar substitutes to increased chronic disorders like cardiovascular disease and diabetes. However, the mechanisms involved were previously unexplored.
This paints a clearer picture of what the researchers may have believed going in. They seemed to accept the earlier claims about artificial sweeteners at face value, assuming there must be some unique mechanism driving those outcomes. Their goal, then, was to fill in the missing piece — to uncover the cellular pathway that might explain why artificial sweeteners appeared to be associated with heart disease.
From that angle, their focus on mechanisms was scientifically reasonable — but their reliance on shaky prior studies was their first misstep. Rather than questioning whether those earlier findings were flawed (which they could have discovered with minimal research), they took them as a given and built their entire experiment on it. It’s like trying to figure out how unicorns metabolize dihydrogen monoxide without first confirming whether unicorns exist.
To their credit, they actually stumbled upon something potentially interesting — the CX3CL1/CX3CR1 pathway that can lead to inflammation. Indeed, this is the mechanism of action, but because they were convinced that aspartame was a unique trigger (rather than just one of many possible triggers of insulin secretion, like, you know, regular sugar), they didn’t really discover anything novel at all.
In the end, they wound up manufacturing a sensationalist narrative instead of a meaningful discovery. And, as expected, when their own data showed that aspartame didn’t induce hyperinsulinemia unless and until it got to unrealistic levels, they should have pivoted. Instead, they leaned into the aspartame angle.
In short, this wasn’t just bad science — it was bad judgment. They were so fixated on proving aspartame’s guilt that they missed their own, more meaningful finding. Their forehead-slapping mistake wasn’t in chasing the wrong hypothesis — that happens all the time in science. The mistake was in doubling down on a bad hypothesis even when their own data disproved it.
Ok, so then why didn’t the journal catch it? They had people review the article to validate it, right?
Sigh – peer review is also imperfect. Reviewers may lack time, expertise in certain areas, or even a full understanding of a paper’s broader implications. The rise in retracted papers is an increasing concern. For studies that are not fraudulent but are retracted due to methodological flaws, misleading conclusions, or overstated claims — such as this paper might be — are common. For example:
A 2018 study in Science analyzed 1,620 retracted papers and found that 21% were retracted due to errors rather than fraud or misconduct. These errors included things like poor experimental design, unreliable data, or conclusions unsupported by evidence.
Another study in Nature reported that error-based retractions are rising, with many stemming from overhyped claims, particularly in high-profile journals that attract media attention.
The website Retraction Watch, which tracks retractions in scientific literature, frequently highlights cases where studies were retracted not for fraud, but for unreliable data, inadequate peer review, or misleading conclusions.
In short, studies like this one in Cell Metabolism likely fall into this more common category: not fraudulent, but deeply flawed in ways that may mislead readers or lead to exaggerated headlines.
When you encounter eyebrow-raising headlines, one of the best strategies is to conduct a Google search like this: “Critical reviews of [Study Title]”
This often reveals insights from other experts who can offer valuable perspectives.
Final Thoughts: Three Healthy Lessons
In short, this study isn’t a cautionary tale about aspartame — it’s a valuable reminder of some important truths:
🔹 Artificial sweeteners are not inherently dangerous, but they are essential tools for diabetics and non-diabetics alike aiming to reduce caloric intake and improve glycemic control.
🔹 Taking too much insulin can increase cardiovascular risk, but so can too much blood sugar. Both can cause an inflammatory response in the cardiovascular system, which can lead to heart disease. Being healthy involves balancing glucose intake with energy expenditure. In other words, favor exercise over insulin to reduce glucose levels, which also leads to a healthy metabolic system. For deeper insights on this, see my article: “The Paradox of Low-Carb Diets: A1c vs. Metabolic Health”
🔹 When you see surprising headlines—good or bad—pause before reacting. Studies come out all the time with claims of curing diabetes, delaying onset, huge benefits of insulin pumps, or the risks of dietary sweeteners. In keeping with the theme of my substack, “It’s not that simple!” Nothing is simple. To get a better understanding of how to understand medical research as a non-scientist, see my article, “Who’s the Grand Wizard of T1D Knowledge?”
Artificial sweeteners have been studied for decades — and despite the persistent alarms, the overwhelming body of research shows that they remain a safe and useful tool for improving metabolic health. For an even deeper dive into this topic, I highly recommend reading Peter Attia’s article on the broader landscape of artificial sweeteners: The Truth About Sugar Substitutes.
Look, if you’re still really afraid of aspartame, there’s nothing better than a cold glass of dihydrogen monoxide.
Cheers!






Phew! Now I can go back to drinking a Diet Coke everyday!
Thank you so much for this post! I was wondering, where in the Attia podcast does Dr. DeFronzo describe that 36unit upper limit?